147118-39-6
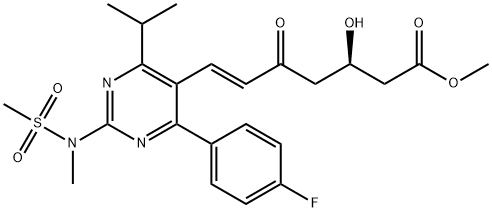 147118-39-6 结构式
147118-39-6 结构式
基本信息
瑞舒伐他汀钙J9
瑞舒伐他汀钙中间体六
瑞舒伐他汀钙中间体J9
瑞舒伐他汀中间体H-2
瑞舒伐他汀5-氧代酸甲酯
5-氧瑞舒伐他汀钙甲酯 标准品
(+)-(3R)-7-[4-(4-氟苯基)-6-异丙基-2-(N-甲基-N-甲磺酰氨基)嘧啶-5-基
(+)-(3R)-7-[4-(4-氟苯基)-6-异丙基-2-(N-甲基-N-甲磺酰氨基)嘧啶-5-基]-3-羟基-5-氧
(R,E)-7-(4-(4-氟苯基)-6-异丙基-2-(N-甲基甲基磺酰氨基)嘧啶-5-基)-3-羟基-5-氧代庚-6-烯酸甲酯
D36-C(GLS)
6(E)-heptenoate
5-Oxorosuvastatin Methyl ester
Rosuvastatin 5-Oxo Acid Methyl Ester
7-[4-fluorophenyl]-6-isopropyl)-2-(N-Methyl-N-Meth...
Methyl(+)-(3-R)-7-[4-(4-Fluorophenyl)-6-isopropyl-2-(N-methy
(R,E)-Methyl 7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)-pyrimidin-5-yl)-3
(R,E)-Methyl 7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)-pyrimidin-5-yl)-3-hy
Methyl(+)-(3-R)-7-[4-(4-Fluorophenyl)-6-isopropyl-2-(N-Methyl-N-Methanesul fonylaMino) pyriMidin-5-y
物理化学性质
制备方法
![7-[4-(4-氟苯基)-6-异丙基-2-(N-甲基甲磺酰胺基)-5-嘧啶]-(3R)-叔丁基二甲硅氧基-5-氧代-(6E)-庚酸甲酯](/CAS/GIF/147118-38-5.gif)
147118-38-5

147118-39-6
在冰浴冷却条件下,将16g 7-[4-(4-氟苯基)-6-异丙基-2-(N-甲基甲磺酰胺基)-5-嘧啶]-(3R)-叔丁基二甲硅氧基-5-氧代-(6E)-庚酸甲酯(化合物ii)溶解于100mL乙腈中。随后,缓慢滴加由48%氟化氢和乙腈按体积比1:19配制的400mL溶液。将反应混合物逐渐升温至室温,并持续搅拌1.5小时。反应完成后,用碳酸氢钠溶液中和反应混合物,随后用乙醚进行萃取。有机层用氯化钠水溶液洗涤,经无水硫酸钠干燥后,减压浓缩除去溶剂。最终,通过蒸馏去除残余乙醚,得到13g(产率:100%)目标产物7-[4-(4-氟苯基)-6-异丙基-2-(N-甲基-N-甲基磺酰氨基)嘧啶-5-基]-(3R)-3-羟基-5-氧代-(E)-6-庚烯酸甲酯(化合物iii),为糖浆状物质。
参考文献:
[1] Patent: WO2006/17357, 2006, A1. Location in patent: Page/Page column 15
[2] Patent: WO2003/97614, 2003, A2. Location in patent: Page/Page column 8; 13
[3] Patent: CN104910078, 2017, B. Location in patent: Paragraph 0012; 0034; 0038-0040; 0046; 0052; 0058; 0064
[4] Bioorganic and Medicinal Chemistry, 1997, vol. 5, # 2, p. 437 - 444
[5] Patent: WO2006/91771, 2006, A2. Location in patent: Page/Page column 26