147126-65-6
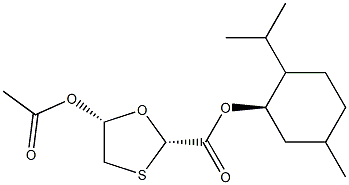 147126-65-6 结构式
147126-65-6 结构式
基本信息
(2R,5S)-L-Menthyl-5-(acetyloxy)-1,3-oxathiolane-2-carboxylate
(2R,5S)-L-Menthol-5-(acetyloxy)-1,3-oxathiolane-2-carboxylate
1,2,5-Menthyl-5(S)-acetoxy-[1,3]-oxathiolene-2-(R)-carboxylate
(5R)-(1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 5-acetoxy-1,3-oxathiolane-2-carboxylate
(1R,2S,5R)-2-Isopropyl-5-Methylcyclohexyl (2R,5S)-5-Acetoxy-1,3-Oxathiolane-2-Carboxylate
(2R,5S)-(1R,2S,5R)-2-Isopropyl-5-Methylcyclohexyl 5-acetoxy-1,3-oxathiolane-2-carboxylate
"2'S-isopropyl-5'R-Methyl-1'R-cyclohexyl ester (2R,5S)-5-acetoxy[1,3]oxathiolane-2-carboxylate
(2R,5S)-5-(Acetyloxy)-1,3-oxathiolane-2-carboxylic Acid (1R,2S,5R)-5-Methyl -2-(1-methylethyl)cyclohexyl Ester
物理化学性质
制备方法

108-24-7
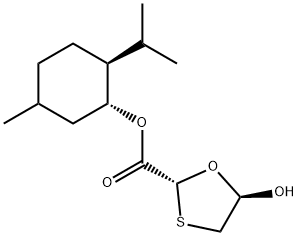
147126-62-3

147126-65-6
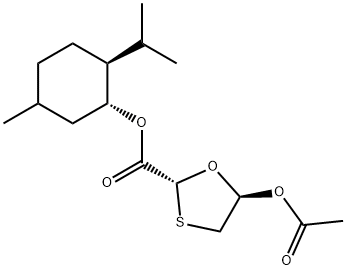
147027-09-6
在0-5℃下,将(1R,2S,5R)-5-甲基-2-异丙基环己基-5R-羟基-1,3-氧硫杂环戊烷-2R-羧酸酯(300g,1.04mol)悬浮于二异丙基醚(1050ml)中,加入吡啶(103.5g,1.31mol)和4-二甲基氨基吡啶(0.35g,3mmol),在氮气保护下搅拌。将乙酸酐(123.6g,1.21mol)用二异丙醚(380ml)稀释后,于0-8℃下在约2小时内滴加到反应混合物中。反应在3-8℃下继续搅拌10小时以确保反应完全(通过TLC监测)。反应完成后,将混合物升温至室温(20-30℃),并用二异丙醚稀释。随后,用5% v/v乙酸水溶液(2×400ml)和温水(2×500ml,约40℃)依次洗涤,以彻底去除吡啶。有机层在低于45℃下减压浓缩,得到固体残余物。向残余物中加入己烷(390ml),加热至约50℃并保持15-20分钟,然后冷却至室温并搅拌30分钟。将产物浆液进一步冷却至-6℃至-10℃,并在该温度下搅拌3小时。过滤收集产物,用预冷的己烷(150ml,-6℃至-8℃)洗涤。产物在约45℃下减压干燥6小时,得到(2R,5S)-(1R,2S,5R)-2-异丙基-5-甲基环己基-5-乙酰氧基-1,3-氧硫杂环戊烷-2-羧酸酯,产量279.6g,HPLC纯度(RI检测器)99.87%。产物为(2R,5R)和(2R,5S)异构体的混合物,其中(2R,5R)为主要成分(≥90%),(2R,5S)为次要成分(≤10%),通过HPLC(RI检测器)分析确定。乙酰氧基化合物的异构体比例对糖苷化反应中α:β异构体形成的比例无显著影响。
参考文献:
[1] Patent: WO2010/82128, 2010, A1. Location in patent: Page/Page column 22
[2] Patent: US2011/282046, 2011, A1. Location in patent: Page/Page column 9
