1489-97-0
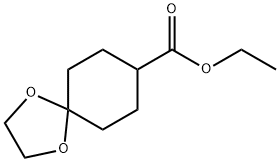 1489-97-0 结构式
1489-97-0 结构式
基本信息
DIN-115244
1,4-二恶螺[4.5]癸烷-8-羧酸乙酯
1,4-二噁螺[4.5]癸烷-8-羧酸乙酯
1,4-二氧杂螺癸烷[4.5]-8-羧酸甲酯
1,4-二氧杂螺[4.5]癸烷-8-羧酸乙酯
1,4-二氧杂螺[4.5]癸烷-8-甲酸 乙酯
1,4-二氧杂-螺环[4,5]癸烷-8-羧酸乙酯
129311
dioxaspiro[4.5]decane-8-carboxyL
dioxaspiro[4.5]decane-8-carboxylate
1,4-Dioxa-8-carboethoxyspiro[4.5]decane
Ethyl 1,4-Dioxaspiro[4.5]decan-8-carboxylate
ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate
Ethyl cyclohexanone-4-carboxylate ethylene ketal
Ethyl 4-Oxocyclohexanecarboxylate Ethylene Ketal
1,4-Dioxaspiro[4.5]decane-8-carboxylic Acid Ethyl Ester
物理化学性质
制备方法
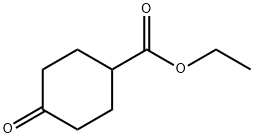
17159-79-4
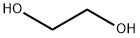
107-21-1
![1,4-二氧杂螺[4.5]癸烷-8-羧酸乙酯](/CAS/GIF/1489-97-0.gif)
1489-97-0
将4-氧代-环己烷甲酸乙酯(52.8 g,0.31 mol)、乙二醇(67.4 g,1.08 mol)和对甲苯磺酸(0.7 g)溶于甲苯(160 mL)中,室温下搅拌反应混合物。反应完成后,将反应溶液倒入乙醚(300 mL)中,依次用水、碳酸氢钠溶液和氯化钠溶液洗涤,无水硫酸钠干燥,减压浓缩,得到无色液体产物。产物未经进一步纯化直接用于下一步反应,产率:66.5 g(100%)。1H-NMR(CDCl3)δ:1.24(t,3H),1.53(m,2H),1.76(m,4H),1.92(m,2H),2.31(m,1H),3.91(s,4H),4.11(q,2H)。13C-NMR(CDCl3)δ:14.28(q),26.32(t),33.76(t),41.59(d),60.14(t),64.21(t),107.90(d),174.77(s)。将仲丁二醇(1.08 mol)和对甲苯磺酸(0.7 g)加入到4-氧代-环己烷羧酸乙酯(0.31 mol)的甲苯(160 mL)溶液中,25℃下搅拌20小时。反应结束后,加入乙酸乙酯(300 mL),分离有机相,依次用水、饱和碳酸氢钠水溶液和氯化钠溶液洗涤,无水硫酸钠干燥,过滤后减压除去溶剂。所得产物无需进一步纯化即可用于下一步反应。
参考文献:
[1] Synthesis, 1998, # 4, p. 436 - 443
[2] Patent: US2008/306084, 2008, A1. Location in patent: Page/Page column 33-34; 44-45
[3] Patent: US2008/312231, 2008, A1. Location in patent: Page/Page column 17
[4] Patent: US2009/48323, 2009, A1. Location in patent: Page/Page column 14; 16
[5] Patent: US2009/111842, 2009, A1. Location in patent: Page/Page column 8
