1563-56-0
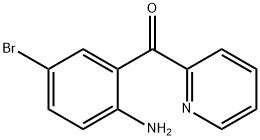 1563-56-0 结构式
1563-56-0 结构式
基本信息
溴西泮杂质A(EP)
溴西EPA EP杂质A
4-溴-2-(吡啶-2-羰基)苯胺
2-(2-氨基-5-溴代苯甲酰)吡啶
2-氨基-5-溴苯基-2-吡啶基甲酮
2-(2-氨基-5-溴-苯甲酰基)吡啶
Bromazepam EP Impurity A
2-amino-5-bromobenzoylpyridine
2-(2-Ao-5-bromobenzoyl) pyridine
2-(5-Bromo-2-aminobenzoyl)pyridine
2-(2-AMino-5-BroMobenzoyl) Pyridin
2-(2-AMINO-5-BROMOBENZOYL) PYRIDINE
Pyridine, 2-(2-amino-5-bromobenzoyl)
2-AMino-5-broMophenyl 2-pyridyl Ketone
4-Bromo-2-[(2-pyridyl)carbonyl]aniline
物理化学性质
制备方法
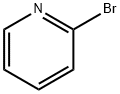
109-04-6
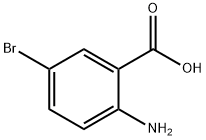
5794-88-7

1563-56-0
以2-溴吡啶和2-氨基-5-溴苯甲酸为原料合成2-(2-氨基-5-溴-苯甲酰基)吡啶的一般步骤:在-40℃下,向2.5M正丁基锂的己烷溶液(400mL,1000mmol,4当量)和乙醚(1L)的混合液中缓慢加入2-溴吡啶(173.93g,1101mmol,4.4当量)。将反应混合物在-40℃下搅拌1小时,然后滴加2-氨基-5-溴苯甲酸(54.14g,250.6mmol,1当量)的四氢呋喃(THF,1L)溶液。将反应体系缓慢升温至0℃,并在该温度下继续搅拌2小时。随后,加入氯三甲基硅烷(625mL,4924mmol,20当量)以淬灭反应。将混合物在室温下搅拌30分钟,然后冷却至0℃,并用3N盐酸(625mL)淬灭。分离水层,有机层用3N盐酸萃取一次。合并的水相用固体氢氧化钠颗粒中和,同时用冰浴冷却。中和后的水相用乙醚(3×1L)萃取。合并的乙醚萃取液用无水硫酸钠干燥,过滤并浓缩,得到黑色油状物。该粗产物通过快速色谱法(硅胶柱,1L,洗脱剂为20-30%乙酸乙酯/己烷)纯化,得到目标化合物2-(2-氨基-5-溴-苯甲酰基)吡啶,为棕色固体(62g,224mmol,收率89.3%)。
参考文献:
[1] Patent: EP1183243, 2006, B1. Location in patent: Page/Page column 11
[2] Patent: WO2014/154762, 2014, A1. Location in patent: Page/Page column 62
[3] Patent: US2014/296230, 2014, A1. Location in patent: Paragraph 0303-0306
[4] Patent: CN108264499, 2018, A. Location in patent: Paragraph 0050-0051; 0075; 0076; 0077-0079
