158985-25-2
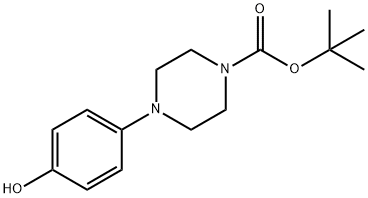 158985-25-2 结构式
158985-25-2 结构式
基本信息
1-BOC-4-(4-羟基苯基)-哌嗪
4-(4-羟苯基)哌嗪-1-羧酸叔丁酯
4-(4-羟基苯基)哌嗪-1-甲酸叔丁酯
叔-丁基 4-(4-羟基苯基)哌嗪-1-甲酸基酯
4-(4-Boc-1-piperazinyl)phenol
N-Boc-4-(4-hydroxyphenyl)piperazine
1-BOC-4-(4-HYDROXY-PHENYL)-PIPERAZINE
4-(4-Hydroxyphenyl)piperazine, N1-Boc protected
tert-Butyl 4-(4-hydroxyphenyl)piperazine-1-carboxylate
4-(4-hydroxyphenyl)-1-piperazinecarboxylic acid tert-butyl ester
1-(4-HYDROXY-PHENYL)-PIPERAZINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER
4-(4-HYDROXY-PHENYL)-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
1-Piperazinecarboxylic acid, 4-(4-hydroxyphenyl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
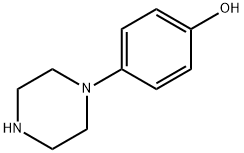
56621-48-8

24424-99-5

158985-25-2
向4-(1-哌嗪基)苯酚(2.0g,11.22mmol)的水(56mL)溶液中加入氢氧化钠(673mg,16.83mmol),随后加入二碳酸二叔丁酯(2.7g,12.34mmol)。将反应混合物在室温下搅拌过夜。次日,过滤反应混合物,收集4-(4-羟基苯基)哌嗪-1-甲酸叔丁酯,为褐色固体(3.1g,收率99%),用水(50mL)洗涤后,不经进一步纯化即可用于下一步反应。1H NMR(300MHz,DMSO-d6):δ6.79(AA'BB',2H,JAB = 9.1Hz,JAA' = 2.4Hz),6.66(AA'BB',2H,JAB = 9.1Hz,JBB' = 2.4Hz),3.43(dd,4H,J = 5.3,4.9Hz),2.88(dd,4H,J = 5.2,5.1Hz),1.41(s,9H);13C NMR(75.5MHz,DMSO-d6):δ153.8,151.4,144.0,118.5,115.4,78.8,50.3,28.0。
参考文献:
[1] Patent: WO2008/83056, 2008, A2. Location in patent: Page/Page column 51
[2] European Journal of Organic Chemistry, 2009, # 25, p. 4290 - 4299
[3] Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 5, p. 1035 - 1049
[4] ACS Medicinal Chemistry Letters, 2017, vol. 8, # 2, p. 215 - 220
[5] Patent: WO2017/93491, 2017, A1. Location in patent: Page/Page column 3; 12
