159603-71-1
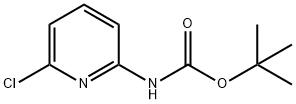 159603-71-1 结构式
159603-71-1 结构式
基本信息
2-叔丁氧羰基氨基-6-氯吡啶
tert-Butyl (6-chloropyridin-2-yl)
2-Amino-6-chloropyridine, 2-Boc protected
ert-Butyl (6-Chloropyridin-2-yl)-carbamate
2-tert-Butoxycarbonylamino-6-chloropyridine
tert-Butyl (6-Chloropyridin-2-yl)-carbamate
tert-Butyl N-(6-chloropyridin-2-yl)carbamate
2-Amino-6-chloropyridine, 2-BOC protected 97%
tert-Butyl (6-Chloropyridin-2-yl)-carbamate ISO 9001:2015 REACH
Carbamic acid, N-(6-chloro-2-pyridinyl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
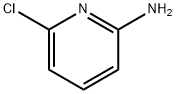
45644-21-1

24424-99-5

159603-71-1
在氮气保护下,将32.7 g (0.15 mol) 二碳酸二叔丁酯(Boc酸酐)溶解于100 mL四氢呋喃(THF)中,制备成溶液。将此溶液缓慢滴加至含有17.4 g (0.14 mol) 2-氨基-6-氯吡啶和300 mL (0.30 mol) 1 M六甲基二硅氮化钠的THF溶液(200 mL THF中)的反应体系中。反应混合物在室温下搅拌过夜。反应完成后,通过旋转蒸发去除溶剂。将残余物在乙酸乙酯(EtOAc)和1 N盐酸水溶液之间分配,充分搅拌后分离有机相。水相再用EtOAc萃取一次。合并所有有机相,用300 mL饱和碳酸氢钠溶液洗涤,随后用无水硫酸钠干燥,过滤后浓缩。粗产物通过从乙醇(EtOH)中重结晶纯化,得到的固体通过抽滤收集并干燥。最终产物为2-Boc-氨基-6-氯吡啶,产量29.2 g,产率95%。产物通过ESI-MS表征:m/z = 228 (M+),HPLC保留时间(Rt)为1.70 min(方法C)。
参考文献:
[1] Patent: US2011/21500, 2011, A1. Location in patent: Page/Page column 45
[2] Patent: US2011/172218, 2011, A1. Location in patent: Page/Page column 29
[3] Patent: US2012/149698, 2012, A1. Location in patent: Page/Page column 26
[4] Journal of Organic Chemistry, 2005, vol. 70, # 5, p. 1771 - 1779
[5] Patent: US2009/258877, 2009, A1. Location in patent: Page/Page column 16-17
