159635-22-0
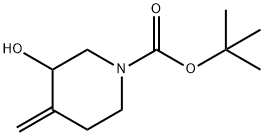 159635-22-0 结构式
159635-22-0 结构式
基本信息
N-BOC-3-羟基-4-亚甲基哌啶
1-叔丁氧羰-3-羟基-4-亚甲基哌啶
3-羟基-4-亚甲基哌啶-1-甲酸叔丁酯
N-Boc-4-methylene-3-piperidinol
N-t-BOC-4-Methylene-3-Piperidinol
N-Boc-3-hydroxy-4-methylenepiperidine
1-Boc-3-hydroxy-4-Methylenepiperidine
tert-butyl3-hydroxy-4-methyleneiperidine-1-carboxylate
tert-Butyl 3-hydroxy-4-Methylenepiperidine-1-carboxylate
tert-butyl 3-hydroxy-4-methylidenepiperidine-1-carboxylate
1-Piperidinecarboxylic acid, 3-hydroxy-4-methylene-, 1,1-dimethylethyl ester
物理化学性质
制备方法
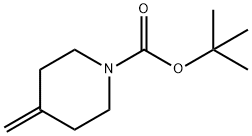
159635-49-1

159635-22-0
以4-亚甲基哌啶-1-羧酸叔丁酯为原料合成3-羟基-4-亚甲基哌啶-1-甲酸叔丁酯的一般步骤:将二氧化硒(2.81 g,0.0253 mol)悬浮于二氯甲烷(150 mL)中,冷却至0℃。缓慢加入叔丁基过氧化氢(13.9 mL,0.101 mol,70%水溶液),混合物在0℃下搅拌30分钟。随后,加入4-亚甲基哌啶-1-羧酸叔丁酯(10.0 g,0.0507 mol)的二氯甲烷(20 mL)溶液,反应混合物在0℃下继续搅拌60分钟,然后升温至23℃搅拌16小时。反应完成后,加入冰和10%亚硫酸氢钠水溶液(150 mL)淬灭反应。分离有机层,水层用二氯甲烷萃取。合并有机相,用无水硫酸镁干燥,过滤并浓缩。通过硅胶柱色谱法(洗脱梯度:25%乙酸乙酯-己烷至50%乙酸乙酯-己烷)纯化,得到目标产物3-羟基-4-亚甲基哌啶-1-甲酸叔丁酯(5.26 g,0.0247 mol,收率49%),为黄色油状物。质谱(FAB-MS):m/e 214。
参考文献:
[1] Journal of Organic Chemistry, 2001, vol. 66, # 7, p. 2487 - 2492
[2] Journal of Organic Chemistry, 2001, vol. 66, # 7, p. 2487 - 2492
[3] Bioorganic and Medicinal Chemistry Letters, 2005, vol. 15, # 5, p. 1375 - 1378
[4] Patent: US2005/182095, 2005, A1. Location in patent: Page/Page column 11
[5] Patent: US2007/10513, 2007, A1. Location in patent: Page/Page column 25
