161157-50-2
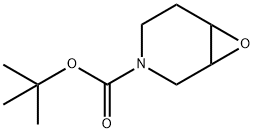 161157-50-2 结构式
161157-50-2 结构式
基本信息
N-BOC-3,4-环氧哌啶
N-BOC-7-氧-3-氮杂双环[4.1.0]庚烷
N-BOC-7-氧杂-3-氮杂双环[4.1.0]庚烷
7-氧杂-3-氮杂双环[4.1.0]庚烷-3-羧酸叔丁酯
7-氧杂-3-氮-二环[4.1.0]庚烷-3-甲酸 叔丁酯
N-BOC-3,4-epoxypiperidine
tert-butyl (1RS,6SR)-7-ox...
7-Oxa-3-azabicyclo[4.1.0]heptan
N-Boc-7-oxa-3-azabicyclo[4.1.0]heptane
1-tert-Butoxycarbonyl-3,4-epoxypiperidine
Tert-butyl 7-oxa-3-azabicyclo[4.1.]heptane-3-carboxylate
tert-butyl 7-oxa-4-azabicyclo[4.1.0]heptane-4-carboxylate
tert-Butyl 7-oxa-3-aza-bicyclo[4.1.0]heptane-3-carboxylate
1,1-Dimethylethyl 7-oxa-3-azabicyclo[4.1.0]heptane-3-carboxylate
物理化学性质
制备方法
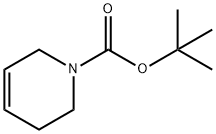
85838-94-4

161157-50-2
以N-Boc-1,2,3,6-四氢吡啶为原料合成7-氧杂-3-氮杂双环[4.1.0]庚烷-3-羧酸叔丁酯的一般步骤如下:按照文献WO 97/10212所述,将5,6-二氢吡啶-1(2H)-羧酸叔丁酯(2.00 g,10.9 mmol)溶解于二氯甲烷(DCM)中,加入间氯过氧苯甲酸(MCPBA,4.16 g,18.6 mmol)。反应混合物在室温下搅拌16小时。反应完成后,依次用饱和焦亚硫酸钠水溶液、饱和碳酸氢钠水溶液、1N氢氧化钠水溶液和饱和盐水洗涤反应混合物。分离有机层,用无水硫酸钠干燥,过滤后减压浓缩,得到7-氧杂-3-氮杂双环[4.1.0]庚烷-3-羧酸叔丁酯(2.04 g,收率94%),为油状物。产物经1H NMR(400 MHz,CDCl3)表征,化学位移δ为1.45(s,9H),1.91(m,1H),2.04(br,1H),3.11(m,1H),3.21(br,1H),3.29(m,1H),3.45(br,1H),3.70(br,1H),3.88(br,1H)。
参考文献:
[1] Tetrahedron Letters, 2004, vol. 45, # 37, p. 6841 - 6845
[2] Organic Process Research and Development, 2014, vol. 18, # 2, p. 321 - 330
[3] Patent: WO2008/11130, 2008, A2. Location in patent: Page/Page column 136-137
[4] Patent: WO2013/138210, 2013, A1. Location in patent: Paragraph 0243
[5] European Journal of Medicinal Chemistry, 2018, vol. 150, p. 39 - 52
