161491-24-3
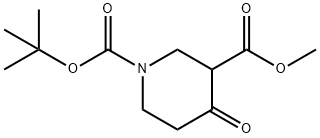 161491-24-3 结构式
161491-24-3 结构式
基本信息
N-BOC-3-甲氧羰基-4-哌啶酮
N-BOC-3-甲酸甲酯-4-哌啶酮
1-BOC-4-氧代-3-哌啶羧酸甲酯
N-BOC-4-氧代-3-哌啶甲酸甲酯
N-BOC-4-氧代哌啶-3-甲酸甲酯
1-叔丁氧羰基-3-甲酸甲酯-4-哌啶酮
4-氧代哌啶-1-甲酸叔丁酯-3-甲酸甲酯
1-叔丁基3-甲基4-哌啶酮-1,3-乙二酸酯
N-BOC-4-氧代哌啶-3-甲酸甲酯(P4M)
Methyl N-Boc-4-piperidone-3-carboxylate
Methyl N-BOC-4-piperidine-3-carboxylate
Methyl 1-BOC-4-oxopiperidine-3-carboxylate
METHYL N-BOC-4-OXOPIPERIDINE-3-CARBOXYLATE
Methyl1-N-Boc4-oxopiperidine-3-carboxylate
Methyl N-Boc-4-Oxopyrrolidine-3-carboxylate
1-N-Boc Methyl 4-oxopiperidine-3-carboxylate
1-Boc-4-oxo-3-piperidine carboxylic acid Methyl ester
1-tert-Butyl 3-methyl 4-oxopiperidine-1,3-dicarboxylate
物理化学性质
制备方法

79099-07-3

616-38-6

161491-24-3
在室温和氮气保护下,将60%氢化钠(3.5g,88mmol)悬浮于无水甲苯(50mL)中。在0.5小时内,向此悬浮液中缓慢滴加碳酸二甲酯(4.32g,48.0mmol)。加入几滴甲醇后,将N-叔丁氧羰基-4-哌啶酮(4.8g,24mmol)溶于无水甲苯(20mL)的溶液滴加到反应混合物中,同时保持反应体系在80℃下搅拌。反应混合物在相同温度下继续搅拌3小时,随后冷却至0℃(冰浴),并用乙酸调节pH至6-6.5。将所得冷混合物用水(10mL)稀释,并用5%氢氧化钠溶液调节pH至8。分离甲苯层,水层用甲苯(20mL)萃取。合并的有机层用无水硫酸钠干燥,减压浓缩。将产物真空干燥,得到甲基-1-叔丁氧基羰基-4-氧代-3-哌啶羧酸甲酯(5.0g,产率80%)。所得化合物无需进一步纯化,可直接用于后续反应。
参考文献:
[1] European Journal of Medicinal Chemistry, 2009, vol. 44, # 10, p. 4063 - 4069
[2] Patent: WO2016/123571, 2016, A1. Location in patent: Paragraph 00265
[3] Journal of the American Chemical Society, 2016, vol. 138, # 40, p. 13271 - 13280
[4] Journal of the American Chemical Society, 2015, vol. 137, # 21, p. 6738 - 6741
[5] Patent: CN107163045, 2017, A. Location in patent: Paragraph 0037; 0038
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | M3338 | 4-氧代哌啶-1,3-二甲酸1-叔丁酯3-甲酯 1-tert-Butyl 3-Methyl 4-Oxopiperidine-1,3-dicarboxylate | 161491-24-3 | 5G | 200元 |
| 2025/12/22 | M3338 | 4-氧代哌啶-1,3-二甲酸1-叔丁酯3-甲酯 1-tert-Butyl 3-Methyl 4-Oxopiperidine-1,3-dicarboxylate | 161491-24-3 | 25G | 660元 |
| 2025/05/22 | XW16149124301 | N-BOC-4-哌啶酮-3-甲酸甲酯 | 161491-24-3 | 25G | 153元 |
