161715-21-5
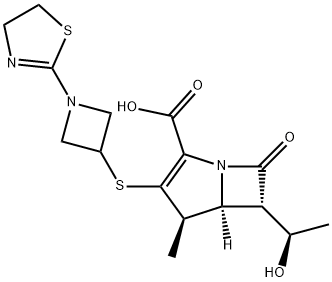 161715-21-5 结构式
161715-21-5 结构式
基本信息
泰比培南
替比培南杂质
泰比培南四水合物
LJC-11036
Tebipenem
LJC 11036
Tebipenem USP/EP/BP
Tebipenem Impurity 5
Tibipenem intermediate 2
(4R,5S,6S)-3-[[1-(4,5-Dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 3-[[1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-, (4R,5S,6S)-
(4R,5S,6S)-3-[1-(4,5-dihydro-1,3-thiazol-2-yl)azetidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylicaci
物理化学性质
制备方法
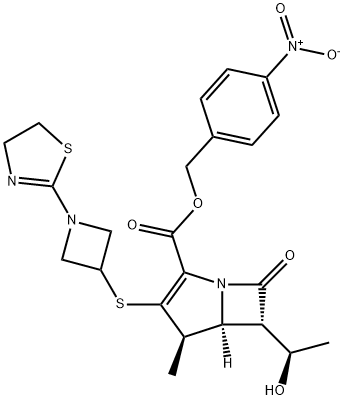
161715-20-4

161715-21-5
以替比培南缩合物为原料合成(4R,5S,6S)-3-((1-(4,5-二氢噻唑-2-基)氮杂环丁烷-3-基)硫基)-6-((R)-1-羟乙基)-4-甲基-7-氧代-1-氮杂双环[3.2.0]庚烷-2-烯-2-羧酸的一般步骤如下:向化合物(II)(R为对硝基苄基)(15.0g,29.0mmol)中加入200mL正丁醇和200mL水,搅拌至完全溶解。随后加入8.00g Raney Ni,在60℃下进行氢化反应。反应在2.0MPa氢气压力、20℃条件下持续4小时。反应完成后,过滤收集固体,并用15mL水洗涤滤饼。滤液用4-二甲基氨基吡啶调节至pH6.5,然后用150mL乙酸乙酯洗涤。将溶液冷却至0℃后,逐滴加入700mL丙酮,搅拌10分钟,再次逐滴加入700mL丙酮,继续搅拌1小时。最终得到12.5g白色固体的替比脒,产率为94.5%,纯度为99.6%。
参考文献:
[1] Patent: CN102757430, 2016, B. Location in patent: Paragraph 0040; 0041
[2] Patent: CN106083858, 2016, A. Location in patent: Paragraph 0042
[3] Journal of Antibiotics, 2006, vol. 59, # 4, p. 241 - 247
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-A0076 | 泰比培南 Tebipenem | 161715-21-5 | 10mg | 140元 |
| 2025/12/22 | HY-A0076 | 泰比培南 Tebipenem | 161715-21-5 | 10mM * 1mLin DMSO | 154元 |
| 2025/12/22 | HY-A0076 | 泰比培南 Tebipenem | 161715-21-5 | 25 mg | 220元 |
常见问题列表
Tebipenem exhibits slow tight-binding inhibition at low micromolar concentrations versus the chromogenic substrate nitrocefin, and apparent K m and k cat values of 0.8 μM and 0.03 min -1 , respectively. Tebipenem shows potent activity against B. pseudomallei , with MIC 50 and MIC 90 values of both 2 mg/L. Tebipenem shows good activity against S. pneumoniae , with the MIC range of ≤0.25 μg/mL in all of the S. pneumoniae isolates.
