164332-88-1
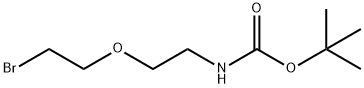 164332-88-1 结构式
164332-88-1 结构式
基本信息
叔丁氧羰基-二聚乙二醇-溴代
N-BOC-2-(2-溴乙氧基)乙胺
(2-(2-溴乙氧基)乙基)氨基甲酸叔丁酯
N-Boc-PEG1-bromide
t-boc-N-amido-PEG2-bromid
t-boc-N-amido-PEG2-bromide
t-boc-N-amido-PEG1-bromide
tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate
tert-Butyl N-[2-(2-bromoethoxy)ethyl]carbamate
Carbamic acid, N-[2-(2-bromoethoxy)ethyl]-, 1,1-dimethylethyl ester
物理化学性质
制备方法
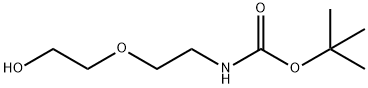
139115-91-6

164332-88-1
以2-(2-BOC-氨基乙氧基)乙醇为原料合成(2-(2-溴乙氧基)乙基)氨基甲酸叔丁酯的一般步骤:将2-(2-BOC-氨基乙氧基)乙醇(1.25 g,6.09 mmol)溶解于二氯甲烷(34 mL)中。将溶液冷却至0℃,随后向溶液中依次加入甲磺酰氯(MsCl,0.80 mL,10.3 mmol,1.7当量)和三乙胺(Et3N,1.9 mL,13.4 mmol,2.2当量)。反应混合物在室温下搅拌3小时后,用丙酮(33 mL)稀释,并加入溴化锂(LiBr,8.9 g,103 mmol,17当量)。将反应混合物在室温下搅拌过夜。反应完成后,减压蒸发溶剂。粗产物用乙酸乙酯(EtOAc)稀释,依次用水和盐水洗涤。有机相用无水硫酸钠(Na2SO4)干燥。将悬浮液通过棉花过滤,滤液真空浓缩。通过快速柱色谱法纯化,使用石油醚/丙酮(85:15→8:2)作为洗脱剂,得到目标产物(2-(2-溴乙氧基)乙基)氨基甲酸叔丁酯(1.60 g,5.95 mmol,产率98%),为无色油状物。1H-NMR(500 MHz,CDCl3):δ 4.91(s,1H),3.78(t,2H),3.56(t,2H),3.47(t,2H),3.33(d,2H),1.55(s,9H)。
参考文献:
[1] Patent: WO2017/46172, 2017, A1. Location in patent: Page/Page column 83; 84
[2] Chemistry - A European Journal, 2014, vol. 20, # 43, p. 13938 - 13944
[3] Patent: JP2016/196447, 2016, A. Location in patent: Paragraph 0041; 0042; 0045
[4] Patent: WO2012/9309, 2012, A1. Location in patent: Page/Page column 42
[5] Patent: WO2016/42080, 2016, A1. Location in patent: Page/Page column 185; 186
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-130503 | 溴-一聚乙二醇-氨基叔丁酯 N-Boc-PEG1-bromide | 164332-88-1 | 100 mg | 100元 |
| 2025/12/22 | HY-130503 | 溴-一聚乙二醇-氨基叔丁酯 N-Boc-PEG1-bromide | 164332-88-1 | 250 mg | 160元 |
| 2025/12/22 | HY-130503 | 溴-一聚乙二醇-氨基叔丁酯 N-Boc-PEG1-bromide | 164332-88-1 | 500 mg | 230元 |
常见问题列表
|
Cleavable
|
PEGs
|
Alkyl/ether
|
PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins.
ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker.
