1666-04-2
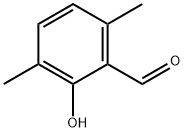 1666-04-2 结构式
1666-04-2 结构式
基本信息
2,6-二甲基-2-羟基苯甲醛
2-羟基-3,6-二甲基苯甲醛
3,6-Dimethylsalicylaldehyde>
3,6-DIMETHYL-2-HYDROXY BENZALDEHYDE
Benzaldehyde, 2-hydroxy-3,6-dimethyl-
3,6-Dimethyl-2-formylphenol, 2-Formyl-3-hydroxy-p-xylene, 3,6-Dimethylsalicylaldehyde
物理化学性质
制备方法
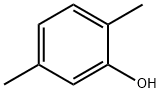
95-87-4
50-00-0

1666-04-2
向2,5-二甲基苯酚(7.0 g,57.3 mmol)的乙腈(250 mL)溶液中依次加入三乙胺(30.1 mL,0.212 mol)和氯化镁(8.17 g,86 mmol)。将反应混合物于室温下搅拌15分钟后,加入多聚甲醛(11.5 g,0.38 mol)。随后,将混合物加热至80℃并保持20小时。反应完成后,通过减压蒸馏除去乙腈。向残余物中加入10%盐酸溶液,室温下搅拌30分钟。用二氯甲烷(2×40 mL)萃取混合物,合并有机层并用无水硫酸钠干燥。减压浓缩有机相,所得粗产物通过硅胶快速柱色谱(洗脱剂:二氯甲烷)纯化,得到目标产物3,6-二甲基-2-羟基苯甲醛(27a)(3.56 g,23.7 mmol,收率42%)。产物结构经1H NMR(400 MHz,CDCl3)确认:δ 2.21(s,3H),2.57(s,3H),6.62(d,J = 8.0 Hz,1H),7.24(d,J = 8.0 Hz,1H),10.30(s,1H),12.18(s,1H)。
参考文献:
[1] Journal of Organometallic Chemistry, 2005, vol. 690, # 23, p. 5125 - 5144
[2] Journal of the Chemical Society, Perkin Transactions 2, 2001, # 9, p. 1573 - 1584
[3] Patent: WO2012/140243, 2012, A1. Location in patent: Page/Page column 137-138
[4] Journal of Organic Chemistry, 2013, vol. 78, # 14, p. 6890 - 6910
[5] Patent: US2016/152543, 2016, A1. Location in patent: Paragraph 1197; 1198; 1199
