167355-41-1
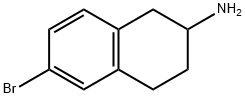 167355-41-1 结构式
167355-41-1 结构式
基本信息
6-bromo-1,2,3,4-tetrahydro-2-Naphthalenamine
6-Bromo-1,2,3,4-tetrahydronaphthalen-2-amine
2-Amino-6-bromo-1,2,3,4-tetrahydronaphthalene
6-Bromo-1,2,3,4-tetrahydro-phthalen-2-ylamine
2-Naphthalenamine, 6-bromo-1,2,3,4-tetrahydro-
6-BROMO-1,2,3,4-TETRAHYDRO-NAPHTHALEN-2-YLAMINE
6-BROMO-1,2,3,4-TETRAHYDRO-NAPHTHALEN-2-YLAMINE HYDROCHLORIDE
物理化学性质
制备方法
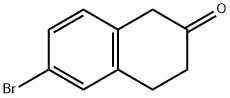
4133-35-1

167355-41-1
一般步骤:制备6-溴-1,2,3,4-四氢萘-2-胺。向含有6-溴-3,4-二氢-1H-2-萘酮(2g,8.89mmol)和NH4OAc(5.52g,71.61mmol)的甲醇(100mL)溶液中,在室温下加入NaCNBH3(0.67g,10.66mmol)。将反应混合物在室温下搅拌20小时,观察到溶液变为黄色。反应完成后,用2M HCl酸化反应混合物,搅拌10分钟,随后蒸发去除甲醇。将混合物用二氯甲烷(CH2Cl2)萃取两次。水层用1.0N NaOH调节至pH 10,再次用二氯甲烷(CH2Cl2)萃取两次。合并有机层,用无水MgSO4干燥,真空浓缩,得到1.31g(65%收率)目标产物6-溴-1,2,3,4-四氢萘-2-胺,为黄色油状物,无需进一步纯化即可用于后续反应。LRMS:m/z 226.1(M + H)+,209(M + H-NH3)+。保留时间:3.84分钟(方法B)。1H NMR(300MHz,CD3OD)δ7.27-7.35(m,8H),7.05(d,J = 8.4Hz,4H),3.56(m,1H),3.17(dd,J = 3.9,16.2Hz,1H),2.95(m,2H),2.81(dd,J = 9.9,16.2Hz,1H),2.19-2.29(m,1H),1.79-1.92(m,1H)。
参考文献:
[1] Patent: EP2202232, 2010, A1. Location in patent: Page/Page column 71
[2] Patent: WO2016/16162, 2016, A1. Location in patent: Page/Page column 21
[3] Patent: WO2016/33025, 2016, A1. Location in patent: Page/Page column 62-63
[4] Patent: WO2008/1160, 2008, A1. Location in patent: Page/Page column 21-23
[5] Patent: US2005/75324, 2005, A1. Location in patent: Page/Page column 9; 10
