169206-55-7
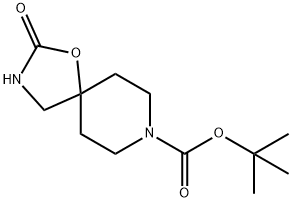 169206-55-7 结构式
169206-55-7 结构式
基本信息
2-氧代-1-噁-3,8-二氮杂螺[4.5]癸烷-8-羧酸叔丁酯
2-氧代-1-氧杂-3,8-二氮杂螺[4.5]癸烷-8-羧酸叔丁酯
叔-丁基 2-氧亚基-1-氧杂-3,8-二氮杂螺[4.5]癸烷-8-甲酸基酯
8-tert-Butoxycarbonyl-1-oxa-3,8-diazaspiro[4.5]decan-2-one
tert-Butyl 2-oxo-1-oxa-3,8-diazaspiro[4.5]decane-8-carboxylate
2-Oxo-1-oxa-3,8-diaza-spiro[45]decane-8-carboxylic acid tert-butyl ester
1-Oxa-3,8-diazaspiro[4.5]decane-8-carboxylic acid, 2-oxo-, 1,1-dimethylethyl ester
物理化学性质
制备方法
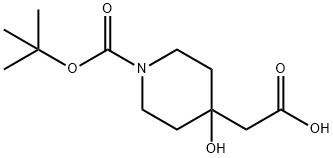
502482-52-2
![2-氧代-1-噁-3,8-二氮杂螺[4.5]癸烷-8-羧酸叔丁酯](/CAS/GIF/169206-55-7.gif)
169206-55-7
以2-(1-(叔丁氧羰基)-4-羟基哌啶-4-基)乙酸为原料合成2-氧代-1-噁-3,8-二氮杂螺[4.5]癸烷-8-羧酸叔丁酯的一般步骤:在室温下,向搅拌的2-(1-(叔丁氧羰基)-4-羟基哌啶-4-基)乙酸(2.1g,8.10mmol)的甲苯(50mL)溶液中加入三乙胺(TEA,884mg,8.75mmol)和二苯基磷酰叠氮(DPPA,3g,10.94mmol)。将反应混合物加热至110℃并搅拌12小时。通过薄层色谱(TLC)监测反应进程;反应完成后,将反应混合物用水(20mL)稀释,并用二氯甲烷(CH2Cl2,2×20mL)萃取。将合并的有机萃取物用无水硫酸钠(Na2SO4)干燥,过滤并减压浓缩,得到粗产物。通过硅胶快速柱色谱法纯化粗产物,用乙酸乙酯(EtOAc)洗脱,得到2-氧代-1-噁-3,8-二氮杂螺[4.5]癸烷-8-羧酸叔丁酯(1.3g,63%),为灰白色固体。1H NMR(500MHz,DMSO-d6):δ7.50(s,1H),3.55-3.51(m,2H),3.30-3.23(m,4H),2.50-1.70(m,2H),1.67-1.62(m,2H),1.39(s,9H);LC-MS纯度:88.4%;(M-Boc)实测值=157.3;(色谱柱:XSelect CSH C-18,50×3.0mm,3.5μm);流速:0.8mL/min;流动相:5mM NH4OAc水溶液:乙腈(ACN)。
参考文献:
[1] Patent: WO2015/48301, 2015, A1. Location in patent: Paragraph 00459
[2] Patent: US2009/105290, 2009, A1. Location in patent: Page/Page column 31
[3] Patent: WO2015/17305, 2015, A1. Location in patent: Page/Page column 64
[4] Patent: WO2016/65582, 2016, A1. Location in patent: Page/Page column 47
[5] Patent: WO2016/60941, 2016, A1. Location in patent: Page/Page column 52