1703-07-7
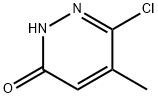 1703-07-7 结构式
1703-07-7 结构式
基本信息
6-氯-5-甲基哒嗪-3-酮
6-氯-5-甲基哒嗪-3(2H)-酮
6-氯-5-甲基-2H-吡嗪-3-酮
6-氯-5-甲基-2H-哒嗪-3-酮
6-chloro-5-methylpyridazine-3-one
3-chloro-4-methyl-1H-pyridazin-6-one
6-Chloro-5-Methylpyridazin-3(2H)-one
6-chloro-5-methyl-2H-pyridazin-3-one
6-Chloro-3-hydroxy-5-methylpyridazine
3(2H)-Pyridazinone, 6-chloro-5-Methyl-
6-Chloro-3-hydroxy-5-methylpyridazine 97%
6-Chloro-5-methylpyridazin-3-ol, 6-Chloro-3-hydroxy-5-methyl-1,2-diazine
物理化学性质
制备方法
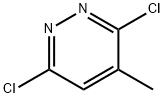
19064-64-3
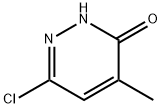
1834-27-1

1703-07-7
在室温下,将3,6-二氯-4-甲基哒嗪(30.6 g,190 mmol)悬浮于冰乙酸(800 mL)中,随后置于110-115°C的油浴中加热3小时(反应1小时后混合物变为均相)。反应完成后,将混合物冷却至室温,并通过减压蒸馏除去乙酸。将得到的固体残余物缓慢加入饱和碳酸氢钠溶液(200 mL)中,得到浅黄色非均相混合物,随后在剧烈搅拌下缓慢加入固体碳酸氢钠调节至pH 6。用二氯甲烷(2×250 mL)萃取混合物,合并有机相后用饱和碳酸氢钠水溶液洗涤,再用氯化钠溶液(60 mL)洗涤,最后用无水硫酸镁干燥并浓缩,得到粗产物(20.9 g)。通过柱色谱法纯化(洗脱梯度:1%甲醇/二氯甲烷至5%甲醇/二氯甲烷),分离得到6-氯-4-甲基-2H-哒嗪-3-酮(13.4 g,92.7 mmol,产率49%)[Rf 0.21(2.5%甲醇/二氯甲烷)]和6-氯-5-甲基-2H-哒嗪-3-酮(5.00 g,34.6 mmol,产率18%)[Rf 0.11(2.5%甲醇/二氯甲烷)],均为无色固体。6-氯-4-甲基-2H-哒嗪-3-酮的核磁共振数据:1H NMR(200 MHz,CDCl3-CD3OD):δ 2.12(3H,s),3.55(1H,br s),7.05(1H,s);13C NMR(50 MHz,CDCl3):δ 16.1(CH3),131.7(CH-5),139.0(C-6),143.0(C-4),161.9(C-3)。6-氯-5-甲基-2H-哒嗪-3-酮的物理和光谱数据:熔点172-173°C(由二氯甲烷/轻质石油醚重结晶);1H NMR(200 MHz,CDCl3):δ 2.20(3H,d,J = 1.3 Hz),7.13(1H,q,J = 1.3 Hz);13C NMR(50 MHz,CDCl3):δ 16.3(CH3),131.9(CH-5),139.2(C-4),143.2(C-6),161.9(C-3)。低分辨质谱(EI):m/z 144([M+])。元素分析计算值(C5H5ClN2O):C,41.54;H,3.49;N,19.38。实测值:C,41.55;H,3.51;N,19.22。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 5, p. 1644 - 1658
[2] Patent: US2012/108578, 2012, A1. Location in patent: Page/Page column 26
[3] Acta Chemica Scandinavica (1947-1973), 1961, vol. 15, p. 1660,1665
[4] Pharmaceutical Bulletin, 1957, vol. 6, p. 587,589
[5] Pharmaceutical Bulletin, 1957, vol. 5, p. 229,233
