171919-36-1
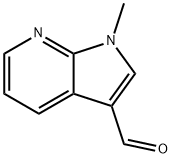 171919-36-1 结构式
171919-36-1 结构式
基本信息
1-Methy-1H-Pyrrolo[2,3-b]pyridine-3-carbaldehyde
1-Methyl-1H-pyrrolo[2,3-b]pyridine-3-carboxaldehyde
1-Methyl-1H-pyrrolo[2,3-b]pyridine-3-carbaldehyde 97%
1-Methyl-1H-Pyrrolo[2,3-B]Pyridine-3-Carbaldehyde(WX624086)
1H-Pyrrolo[2,3-b]pyridine-3-carboxaldehyde, 1-methyl- (9CI)
制备方法
27257-15-4

68-12-2
![1H-Pyrrolo[2,3-b]pyridine-3-carboxaldehyde, 1-methyl- (9CI)](/CAS/GIF/171919-36-1.gif)
171919-36-1
b) 1-甲基-1H-吡咯并[2,3-b]吡啶-3-甲醛的合成: 在0℃下,将三氯氧磷(1.2g, 7.9mmol)缓慢滴加至10mL N,N-二甲基甲酰胺中,同时搅拌。滴加完毕后,继续搅拌10分钟。随后,在1分钟内加入1-甲基-7-氮杂吲哚(0.95g, 7.2mmol)溶于5mL N,N-二甲基甲酰胺的溶液。反应混合物在0℃下搅拌1小时,随后升温至60℃继续搅拌30分钟。反应完成后,将混合物倒入水中,用碳酸氢钠水溶液调节至碱性,并用乙酸乙酯萃取三次。合并有机层,用水洗涤,无水硫酸钠干燥,过滤后减压浓缩。得到粗产物0.85g,收率74%,纯度满足后续反应要求。1H NMR (300MHz, CDCl3) δ: 9.99 (s, 1H), 8.55 (m, 1H), 8.44 (m, 1H), 7.84 (s, 1H), 7.27 (m, 1H), 3.97 (s, 3H)。
参考文献:
[1] Chemical and pharmaceutical bulletin, 1995, vol. 43, # 8, p. 1351 - 1357
[2] Patent: WO2005/66132, 2005, A1. Location in patent: Page/Page column 66
[3] Heteroatom Chemistry, 2011, vol. 22, # 2, p. 148 - 157
[4] Patent: WO2009/3003, 2008, A2. Location in patent: Page/Page column 61-62
[5] Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 3, p. 1201 - 1212
