17295-12-4
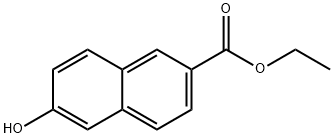 17295-12-4 结构式
17295-12-4 结构式
基本信息
6-羟基-2-萘酸乙酯
2-羟基-6-萘甲酸乙酯
Ethyl6-hydroxy-2-naphtoate
ETHYL 6-HYDROXY-2-NAPHTHOATE
ethyl 6-hydroxynaphthalene-2-carboxylate
2-Naphthalenecarboxylic acid, 6-hydroxy-, ethyl ester
物理化学性质
制备方法

64-17-5
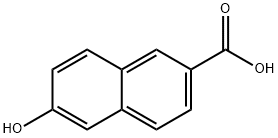
16712-64-4

17295-12-4
1. 在0℃条件下,将6-羟基-2-萘甲酸(5.29 g,28.0 mmol)溶于乙醇(50 mL)中,缓慢加入亚硫酰氯(6 mL)。2. 将反应混合物在室温下搅拌72小时。3. 反应完成后,通过旋转蒸发仪除去过量的试剂。4. 将残余物溶解于乙酸乙酯(100 mL)中,依次用水(80 mL)、冷的1M碳酸氢钠溶液(80 mL)和盐水(80 mL)洗涤。5. 有机相用无水硫酸钠干燥,过滤后浓缩,得到粗产物6-羟基-2-萘酸乙酯,收率99%。6. 产物经1H NMR(CDCl3)和LCMS(APCl)表征:1H NMR(CDCl3):δ8.53(1H,br s),8.01(1H,dd,J = 8.5,1.7 Hz),7.86(1H,d,J = 8.5 Hz),7.20-7.13(2H,m),4.43(2H,q,J = 7.3 Hz),1.43(3H,t,J = 7.1 Hz)。LCMS(APCl):217.1(M + H +)。
参考文献:
[1] Patent: WO2006/40646, 2006, A1. Location in patent: Page/Page column 97
[2] Journal of Physical Chemistry A, 2016, vol. 120, # 33, p. 6563 - 6574
[3] Journal of Medicinal Chemistry, 2004, vol. 47, # 14, p. 3518 - 3536
[4] Molecular Crystals and Liquid Crystals, 2008, vol. 494, p. 282 - 292
[5] Tetrahedron, 2013, vol. 69, # 43, p. 9045 - 9055