179557-01-8
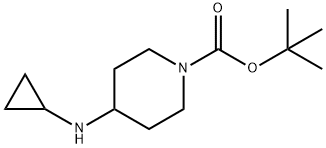 179557-01-8 结构式
179557-01-8 结构式
基本信息
4-(环丙基氨基)哌啶-1-羧酸叔丁酯
1-叔丁氧羰基-4-(环丙基氨基)哌啶
1-叔丁氧基羰基-4-(环丙基氨基)哌啶
1-N-BOC 4-(CYCLOPROPYLAMINO) PIPERIDINE
4-(Cyclopropylamino)piperidine, N1-BOC protected
4-(Cyclopropylamino)piperidine,N1-BOCprotected97%
4-(Cyclopropylamino)piperidine, N1-BOC protected 97%
1-TERT-BUTOXYCARBONYL-4-(CYCLOPROPYLAMINO)PIPERIDINE
4-(Cyclopropylamino)piperidine, N1-BOC protected 95+%
TERT-BUTYL 4-(CYCLOPROPYLAMINO)PIPERIDINE-1-CARBOXYLATE
4-Cyclopropylamino-piperidine-1-carboxylic acid tert-butyl ester
1-Piperidinecarboxylic acid, 4-(cyclopropylamino)-, 1,1-dimethylethyl ester
物理化学性质
安全数据
制备方法

79099-07-3
765-30-0

179557-01-8
向反应瓶中加入4-氧代哌啶-1-羧酸叔丁酯(5.0 g,25.1 mmol)的甲醇(40 mL)溶液,随后加入环丙胺(1.4 g,25.1 mmol)、三乙胺(10.0 mL,75.3 mmol)和氯化锌(0.3 g,2.5 mmol)。将反应混合物在60℃下搅拌7小时,然后分批加入氰基硼氢化钠(4.8 g,75.3 mmol)。反应混合物在25℃下继续搅拌17小时。反应完成后,真空除去溶剂,将残余物在水(250 mL)和乙酸乙酯(200 mL)之间分配。水层用乙酸乙酯(2×200 mL)进一步萃取,合并有机层,用硫酸钠干燥后真空浓缩。通过柱色谱法(碱性活性氧化铝,洗脱剂:10%至30%乙酸乙酯的己烷溶液)纯化残余物,得到目标产物4-(环丙基氨基)哌啶-1-羧酸叔丁酯(5.3 g,收率88%),为胶状物。产物数据见表2。
参考文献:
[1] Patent: WO2017/21730, 2017, A1. Location in patent: Page/Page column 46
[2] Journal of Medicinal Chemistry, 1996, vol. 39, # 19, p. 3769 - 3789
[3] Patent: WO2010/52255, 2010, A1. Location in patent: Page/Page column 53-54
[4] Patent: WO2007/16610, 2007, A2. Location in patent: Page/Page column 57; 58
[5] Patent: EP3147283, 2017, A1. Location in patent: Paragraph 0106
