181269-70-5
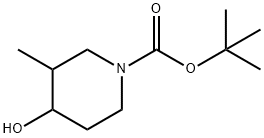 181269-70-5 结构式
181269-70-5 结构式
基本信息
1-BOC-3-甲基-4-羟基哌啶
4-羟基-3-甲基哌啶-1-羧酸叔丁酯
N-叔丁氧羰基-3-甲基-4-羟基哌啶
1-BOC4-羟基-(+)-3-甲基哌啶
N-Boc-3-methyl-4-hydroxypiperidine
tert-Butyl 4-hydroxy-3-Methylpiperidine-1-carboxylate
4-Hydroxy-3-methyl-piperidine-1-carboxylic acid tert-butyl ester
1-Piperidinecarboxylic acid, 4-hydroxy-3-Methyl-, 1,1-diMethylethylester
物理化学性质
制备方法
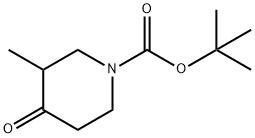
181269-69-2

181269-70-5
以N-Boc-3-甲基-哌啶-4-酮为原料合成4-羟基-3-甲基哌啶-1-羧酸叔丁酯的一般步骤如下: (i)在0℃下,将硼氢化钠(0.265g,7.03mmol)分批加入(R,S)-叔丁基-3-甲基-4-氧代哌啶-1-甲酸酯(1.0g,4.68mmol)的甲醇(10mL)溶液中。保持0℃搅拌反应混合物1小时,随后升温至室温继续搅拌2.5小时。反应完成后,用饱和氯化铵水溶液淬灭反应,减压浓缩除去甲醇。用二氯甲烷萃取反应混合物,合并有机层并用无水硫酸钠干燥。过滤后,减压浓缩有机层,得到无色油状的目标产物4-羟基-3-甲基哌啶-1-羧酸叔丁酯(定量收率)。产物的结构通过1H NMR(400MHz,CDCl3)和质谱(ES+)确认:1H NMR δppm 0.87-0.94(m,3H),1.33-1.88(m,3H),1.38(s,9H),2.23-4.08(m,5H);MS:ES+ 216。
参考文献:
[1] Patent: US2013/324576, 2013, A1. Location in patent: Paragraph 0625; 0626; 0627; 0628
[2] Patent: WO2013/179024, 2013, A1. Location in patent: Page/Page column 56-57
[3] Patent: US2005/182095, 2005, A1. Location in patent: Page/Page column 38
[4] Patent: WO2004/41777, 2004, A2. Location in patent: Page 74-75
[5] Patent: WO2013/96744, 2013, A1. Location in patent: Page/Page column 199
