182223-54-7
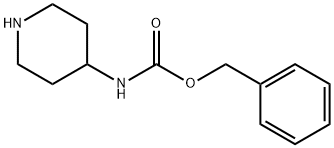 182223-54-7 结构式
182223-54-7 结构式
基本信息
4-(N-苄氧羰基)-氨基哌啶
4-(Z-amino)-piperidine
N-CBZ-piperidin-4-aMine
Benzylpiperidin-4-ylcarbamate
Benzyl 4-Piperidinylcarbamate
4-Benzyloxycarbonylamino-piperdine
benzyl N-(piperidin-4-yl)carbaMate
4-BENZYLOXYCARBONYLAMINO-PIPERIDINE
benzyl piperidin-4-ylcarbamate hydrochloride
Carbamic acid, 4-piperidinyl-, phenylmethyl ester
物理化学性质
制备方法
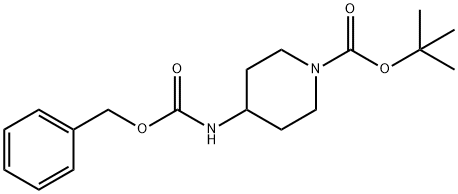
220394-97-8

182223-54-7
以1-Boc-4-(Cbz-氨基)哌啶为原料合成4-(N-苄氧羰基)-氨基哌啶的一般步骤如下:将实施例1a中得到的4-{[(苄氧基)羰基]氨基}哌啶-1-羧酸叔丁酯(13.1g,39.2mmol)与5N盐酸水溶液(40ml,200mmol)和甲醇(40ml)的混合物在室温下搅拌23小时。反应完成后,在冰浴冷却条件下,向反应混合物中缓慢加入5N氢氧化钠水溶液(40ml)。随后,通过蒸馏去除反应混合物中的水和溶剂,同时采用乙醇进行共沸蒸馏以彻底除去水分。向蒸馏后的残余物中加入乙醇,过滤以除去不溶物,浓缩滤液,最终得到4-(N-苄氧羰基)-氨基哌啶(9.10g,收率99.1%)。产物经1H-NMR(400MHz,CDCl3)表征,化学位移δppm如下:1.31-1.42(2H,m),1.92-2.04(2H,br),2.70(2H,d,J = 12Hz),3.10(2H,d,J = 12Hz),3.56-3.68(1H,br),4.72-4.81(1H,br),5.09(2H,s),7.26-7.40(5H,m)。
参考文献:
[1] Patent: US2013/65925, 2013, A1. Location in patent: Paragraph 0197; 0198
[2] Patent: EP2757103, 2014, A1. Location in patent: Paragraph 0149
[3] Journal of Medicinal Chemistry, 2012, vol. 55, # 3, p. 1021 - 1046
[4] Patent: WO2011/60321, 2011, A1. Location in patent: Page/Page column 115
[5] Patent: WO2006/99884, 2006, A1. Location in patent: Page/Page column 18
