182618-86-6
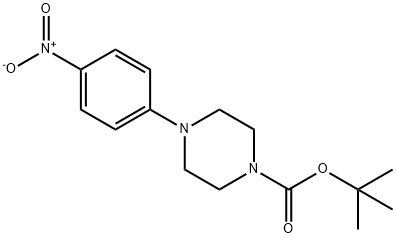 182618-86-6 结构式
182618-86-6 结构式
基本信息
1-BOC-4-(4-硝基苯基)哌嗪基
4-(4-硝基苯基)哌嗪-1-羧酸叔丁酯
1-叔丁氧羰基-4-(4-硝基苯基)哌嗪
4-(4-硝基苯基)-1-哌嗪羧酸叔丁酯
1-BOC-4-(4-NITROPHENYL)-PIPERAZINE
t-Butyl 4-(4-nitrophenyl)piperazine-1-carboxylate
ert-butyl 4-(4-nitrophenyl)piperazine-1-carboxylate
tert-Butyl 4-(4-nitrophenyl)piperazine-1-carboxylate
4-(4-nitrophenyl)-1-piperazinecarboxylic acid tert-butyl ester
4-(4-Nitrophenyl)piperazine-1-carboxylicacidtert-butylester95%
4-(4-NITROPHENYL)PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
TERT-BUTYL 4-(4-NITROPHENYL)TETRAHYDRO-1(2H)-PYRAZINECARBOXYLATE
4-(4-NITROPHENYL)PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER 95%
物理化学性质
制备方法
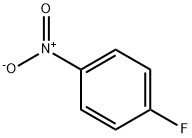
350-46-9
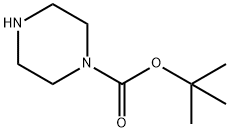
57260-71-6

182618-86-6
以1-氟-4-硝基苯(5g,35.43mmol)和N-BOC-哌嗪(6.6g,35.43mmol)为原料,在N,N-二甲基甲酰胺(DMF,100mL)中溶解,加入碳酸钾(14.7g,106.29mmol)作为碱。将反应混合物在50℃下搅拌18小时。反应完成后,将混合物冷却至室温,随后在减压条件下浓缩以去除溶剂。得到的油状残余物用乙醚洗涤三次,以纯化产物。最终得到4-(4-硝基苯基)哌嗪-1-羧酸叔丁酯,为黄色固体(8.2g,收率95%)。产物经1H NMR(400MHz,氯仿-d)确认:δ 1.48(s,9H),3.38-3.45(m,4H),3.56-3.63(m,4H),6.75-6.86(m,2H),8.07-8.20(m,2H)。质谱(MS)分析显示m/z 308.1([M+H]+),与预期分子式C15H21N3O4相符。
参考文献:
[1] Patent: WO2006/38039, 2006, A1. Location in patent: Page/Page column 17; 30
[2] Patent: WO2017/123542, 2017, A1. Location in patent: Page/Page column 398; 399
[3] Patent: CN106905245, 2017, A. Location in patent: Paragraph 0314; 0315; 0316; 0317; 0318
[4] Patent: US2003/13708, 2003, A1
[5] Patent: US2004/87575, 2004, A1
