18267-36-2
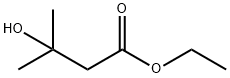 18267-36-2 结构式
18267-36-2 结构式
基本信息
乙基3-羟基-3-甲基丁酸酯
(CH3)2C(OH)CH2C(O)OC2H5
ethyl 3-hydroxy-3-methylbutyrate
ethyl 3-hydroxy-3-Methylbutanoate
3-Hydroxy-3-methylbutanoic acid ethyl ester
Butanoic acid,3-hydroxy-3-Methyl-, ethyl ester
物理化学性质
制备方法

141-78-6

67-64-1

18267-36-2
步骤A:3-羟基-3-甲基丁酸乙酯的合成:在氮气保护下,将双(三甲基甲硅烷基)氨化锂(1M THF溶液,100mL,100mmol)溶于四氢呋喃(100mL)中,冷却至-78℃。缓慢加入乙酸乙酯(9.74mL,100mmol),反应混合物在此温度下搅拌30分钟。随后,加入丙酮(8.81mL,120mmol),继续搅拌10分钟。反应完成后,用2M盐酸水溶液(70mL,140mmol)淬灭反应,并逐渐升温至室温。反应混合物用乙酸乙酯(2×150mL)萃取,合并有机相,依次用饱和碳酸氢钠水溶液(2×50mL)洗涤,无水硫酸镁干燥,过滤后减压浓缩,得到黄色油状目标产物3-羟基-3-甲基丁酸乙酯(12.8g,收率88%)。产物经1H NMR(CDCl3)表征:δ4.18(q,3H),2.49(s,2H),1.29(m,9H)。
参考文献:
[1] Patent: WO2014/78322, 2014, A1. Location in patent: Paragraph 00443
[2] Patent: WO2014/78325, 2014, A1. Location in patent: Paragraph 00627
[3] Patent: WO2014/78408, 2014, A1. Location in patent: Paragraph 00558
[4] Patent: WO2014/78328, 2014, A1. Location in patent: Paragraph 00555
[5] Patent: WO2014/78331, 2014, A1. Location in patent: Paragraph 00756
