188715-40-4
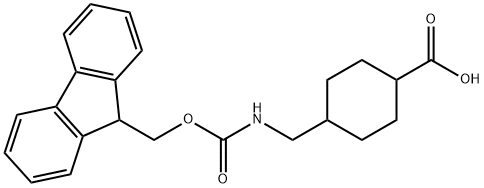 188715-40-4 结构式
188715-40-4 结构式
基本信息
4-(FMOC-氨基乙基)环己烷-1-羧酸
4-[[[芴甲氧羰基]氨基]甲基]环己烷羧酸
4-(FMOC-氨基乙基)环己烷-1-羧酸,98%
FMOC-4-AMC-OH
Fmoc-4-Amc-OH(cis/trans mixture)
Glutamic Acid Impurity 5 Monomer
FMOC-(4-AMINOMETHYL)-CYCLOHEXANE CARBOXYLIC ACID
4-[(Fmoc-amino)methyl]cyclohexanecarboxylic acid
4-(Fmoc-aminomethyl)cyclohexane-1-carboxylic acid
4-(Fmoc-aminomethyl)cyclohexane-1-carboxylicacid,98%
Fmoc-4-aminomethylcyclohexane carboxylic acid≥ 99% (HPLC)
TRANS-4-(9-FLUORENYLMETHYLOXYCARBONYLAMINOMETHYL)-CYCLOHEXANOIC ACID
物理化学性质
制备方法
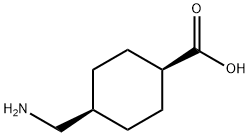
1197-17-7
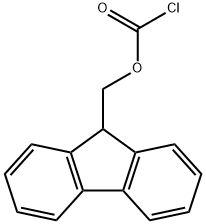
28920-43-6

188715-40-4
步骤1:将cis-4-(氨基甲基)环己基-1-甲酸(1.15 g,7.30 mmol)溶于1,4-二恶烷(12.0 mL)和10% Na2CO3水溶液(23.2 mL)中,随后加入氯甲酸-9-芴基甲酯(Fmoc-Cl,2.26 g,8.76 mmol)。反应混合物在室温下搅拌2.5小时。反应完成后,向混合物中加入1M HCl(42.0 mL),并用乙酸乙酯(EtOAc)萃取三次。合并的有机层依次用1M HCl、水和盐水洗涤。水层再次用EtOAc萃取,合并所有有机层,用无水Na2SO4干燥。减压除去溶剂,得到粗产物。粗产物用冰冷的EtOAc洗涤,并在高真空下干燥,得到N-Fmoc-4-氨甲基环己烷羧酸(2.28 g,产率83%)为白色固体,无需进一步纯化。[参考文献:M. Nichifor; E. H. Schacht; Tetrahedron; 1994; 50; 12; 3747-3760]。1H NMR(400 MHz,CDCl3):δ 0.87-1.02(br.m,2H),1.34-1.52(br.m,4H),1.81(br.d,2H,J = 6 Hz),1.90-2.11(br.s,1H),2.12-2.35(br.m,1H),3.03(Ψt,2H,J = 6.2 Hz),4.19(br.Ψt,1H,J = 6.5 Hz),4.42(br.Ψd,2H,J = 6.5 Hz),4.74(br.s,1H),7.30(Ψt,2H,J = 7.4 Hz),7.38(Ψt,2H,J = 7.4 Hz),7.57(d,2H,J = 7.4 Hz),7.75(d,2H,J = 7.4 Hz)。
参考文献:
[1] Patent: EP1577289, 2005, A1. Location in patent: Page/Page column 23
[2] Organic Letters, 2008, vol. 10, # 10, p. 1881 - 1884
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/09/19 | H66266 | 4-(Fmoc-氨基乙基)环己烷-1-羧酸, 98% 4-(Fmoc-aminomethyl)cyclohexane-1-carboxylic acid, 98% | 188715-40-4 | 5g | 3024元 |
| 2025/05/22 | H66266 | 4-(Fmoc-氨基乙基)环己烷-1-羧酸, 98% 4-(Fmoc-aminomethyl)cyclohexane-1-carboxylic acid, 98% | 188715-40-4 | 1g | 724元 |
| 2024/04/30 | H66266 | 4-(Fmoc-氨基乙基)环己烷-1-羧酸, 98% 4-(Fmoc-aminomethyl)cyclohexane-1-carboxylic acid, 98% | 188715-40-4 | 250mg | 339元 |