192725-45-4
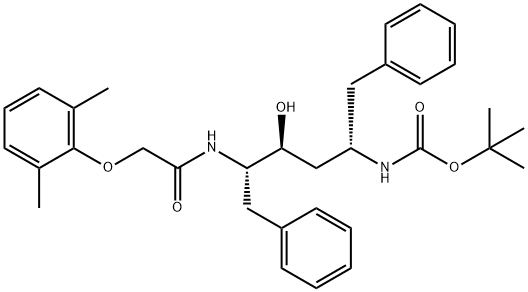 192725-45-4 结构式
192725-45-4 结构式
基本信息
(2S,3S,5S)-2-(2,6-二甲基苯氧乙酰基)氨基-3-羟基-5-(叔丁氧羰基)氨基-1,6-二苯基己烷
tert-Butyl ((2S,4S,5S)-5-(2-(2,6-dimethylphenoxy)acetamido)-4-hydroxy-1,6-diphenylhexan-2-yl)c
tert-Butyl ((2S,4S,5S)-5-(2-(2,6-dimethylphenoxy)acetamido)-4-hydroxy-1,6-diphenylhexan-2-yl)carb
tert-Butyl ((2S,4S,5S)-5-(2-(2,6-dimethylphenoxy)acetamido)-4-hydroxy-1,6-diphenylhexan-2-yl)carbamate
(2S,3S,5S)-2-(2,6-DIMETHYLPHENOXYACETYL)AMINO-3-HYDROXY-5-(T-BUTYLOXYCARBONYLAMINO)-1,6-DIPHENYLHEXANE
(2S,3S,5S)-2-(2,6-Dimethylphenoxyacetyl)amino-3-hydroxy-5-(tert-butoxycarbonyl)amino-1,6-diphenylhexane
tert-butyl N-[(2S,4S,5S)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]carbamate
N-[(1S,3S,4S)-4-[[2-(2,6-DiMethylphenoxy)acetyl]aMino]-3-hydroxy-5-phenyl-1-(phenylMethyl)pentyl]carbaMic Acid 1,1-DiMethylethyl Ester
Carbamic acid, N-[(1S,3S,4S)-4-[[2-(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]-, 1,1-dimethylethyl ester
物理化学性质
制备方法
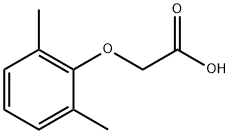
13335-71-2
![[(1S,3S,4S)-4-氨基-3-羟基-5-苯基-1-(苯甲基)戊基]-氨基甲酸叔丁酯](/CAS/GIF/144163-85-9.gif)
144163-85-9

192725-45-4
实施例18. (2S,3S,5S)-2-(2,6-二甲基苯氧乙酰基)氨基-3-羟基-5-(叔丁氧羰基)氨基-1,6-二苯基己烷的制备:在室温下,将(1S,3S,4S)-1-苄基-4-氨基-3-羟基-5-苯基戊基氨基甲酸叔丁酯(38.5 mg, 0.1 mmol)、2,6-二甲基苯氧基乙酸(18.9 mg, 1.05当量)、1-(3-二甲基氨基丙基)-3-乙基碳二亚胺盐酸盐(29.7 mg, 1.5当量)和1-羟基苯并三唑(20.4 mg, 1.5当量)溶于N,N-二甲基甲酰胺(1 mL)中,搅拌4分钟。随后,向反应混合物中加入N-甲基吗啉(27.5 μL, 2.5当量),继续搅拌16小时。反应完成后,用饱和碳酸氢钠溶液淬灭反应,用乙酸乙酯萃取。有机相用10%柠檬酸溶液洗涤,无水硫酸钠干燥,减压浓缩。通过硅胶柱色谱(3%甲醇/二氯甲烷)纯化,得到白色固体产物(240 mg, 76.7%收率)。1H NMR (300 MHz, DMSO-D6) δ ppm: 1.31 (s, 9H), 1.39-1.55 (m, 2H), 2.14 (s, 6H), 2.61 (d, J = 6.99 Hz, 2H), 2.80 (d, J = 7.35 Hz, 2H), 3.61-3.70 (m, 1H), 3.84 (m, 1H), 4.00-4.11 (m, 2H), 4.20-4.38 (m, 1H), 4.99 (d, 1H), 6.66 (d, J = 9.19 Hz, 1H), 6.88-7.28 (m, 13H), 7.43 (d, J = 9.56 Hz, 1H); MS m/z 547.4 (M + H)+。
参考文献:
[1] Patent: EP1170289, 2002, A2. Location in patent: Page 41
[2] Patent: WO2008/27932, 2008, A2. Location in patent: Page/Page column 101
[3] Bioorganic and Medicinal Chemistry Letters, 2002, vol. 12, # 21, p. 3101 - 3103
[4] Letters in Organic Chemistry, 2018, vol. 15, # 2, p. 87 - 91
