192869-80-0
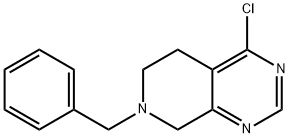 192869-80-0 结构式
192869-80-0 结构式
基本信息
N-苄基-4-氯-5,6,7,8- 四氢吡啶并[3,4-D]嘧啶
7-苄基-4-氯-5-甲基-5,6,7,8-四氢吡啶并[3,4-D]嘧啶
7-Benzyl-4-chloro-5,6,7,8-tetrahydro-quinazoline
7-benzyl-4-chloro-5H,6H,7H,8H-pyrido[3,4-d]pyrimidine
7-BENZYL-4-CHLORO-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIDINE
7-Benzyl-5,6,7,8-tetrahydro4-chloro-pyrido[3,4-D]pyriMidine
7-Benzyl-4-chloro-5,6,7,8-tetrahydro-1,3,7-triazanaphthalene
7-BENZYL-4-CHLORO-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE HCL
Pyrido[3,4-d]pyriMidine,4-chloro-5,6,7,8-tetrahydro-7-(phenylMethyl)-
7-BENZYL-5,6,7,8-TETRAHYDRO4-CHLORO-PYRIDO[3,4-D]PYRIMIDINE HYDROCHLORIDE
7-BENZYL-5,6,7,8-TETRAHYDRO4-CHLORO-PYRIDO[3,4-D]PYRIMIDINE HYDROCHLORIDE ISO 9001:2015 REACH
物理化学性质
制备方法
![7-苄基-5,6,7,8-T四氢吡啶并[3,4-D]嘧啶-4(3H)-酮](/CAS/GIF/62458-96-2.gif)
62458-96-2
![7-苄基-4-氯-5,6,7,8-四氢吡啶并[3,4-D]嘧啶](/CAS/GIF/192869-80-0.gif)
192869-80-0
以7-苄基-5,6,7,8-四氢吡啶并[3,4-D]嘧啶-4(3H)-酮(3.00g,12.4mmol)为原料,在室温下将其溶于甲苯(40mL)中。向该溶液中依次加入三氯氧化磷(2.28g,14.9mmol)和Hunig碱(1.28g,9.92mmol),随后将反应体系加热至100℃并维持30分钟。反应完成后,冷却至室温。将反应混合物用200mL饱和碳酸氢钠水溶液和200mL碎冰淬灭,室温下搅拌1小时。随后,用二氯甲烷(100mL×4)进行萃取。合并有机相,经无水硫酸钠干燥后,减压浓缩,得到3.32g 7-苄基-4-氯-5,6,7,8-四氢吡啶并[3,4-D]嘧啶(69B),为深棕色油状物,产率约100%。该产物无需进一步纯化即可用于下一步反应。HPLC保留时间:0.95分钟。质谱(MH+)m/z:260。
参考文献:
[1] Patent: WO2005/42537, 2005, A1. Location in patent: Page/Page column 86
[2] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 18, p. 5019 - 5024
[3] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 1, p. 160 - 167
[4] Patent: WO2009/158396, 2009, A1. Location in patent: Page/Page column 67-68
[5] Patent: WO2005/66171, 2005, A1. Location in patent: Page/Page column 59-60