193902-78-2
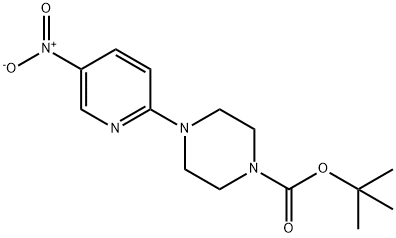 193902-78-2 结构式
193902-78-2 结构式
基本信息
帕博西尼杂质102
帕博西尼杂质SMA-7
PALBOCICLIB 杂质 97
PALBOCICLIB 杂质 102
2-(4-BOC-1-哌嗪基)-5-硝基吡啶
1-BOC-4-(5-硝基-2-吡啶基)哌嗪
1-叔丁基羰基-4-(5-硝基-2-哌啶)哌嗪
1-叔丁氧羰基-4-(5-硝基吡啶-2-基)哌嗪
4-(5-硝基吡啶-2-基) 哌嗪-1-羧酸叔丁酯
Palbociclib Impurity SMA-7
1-Boc-4-(5-nitro-2-pyridinyl)-piperazine
1-Boc-4-(5-nitro-2-pyridyl)piperazine 97%
tert-Butyl 4-(5-nitropyridin-2-yl)piperazine-1-carboxylate
4-(5-nitro-pyridin-2-yl)-piperazine-1-carboxylic acid tert-butyl ester
1-Piperazinecarboxylic acid, 4-(5-nitro-2-pyridinyl)-, 1,1-dimethylethyl ester
1-tert-Butoxycarbonyl-4-(5-nitropyrid-2-yl)piperazine, 2-(4-Boc-1-piperazinyl)-5-nitropyridine
物理化学性质
制备方法
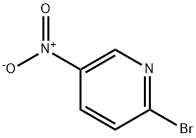
4487-59-6
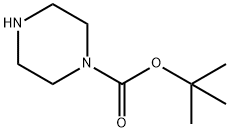
57260-71-6

193902-78-2
一般步骤:将2-溴-5-硝基吡啶(11.39 g,56.1 mmol)、四丁基碘化铵(TBAI)(1.04 g,0.05 mmol)、碳酸钾(8.53 g,61.7 mmol)和N-BOC-哌嗪(11.5 g,61.7 mmol)溶于DMSO(100 mL)中,温和加热至50℃反应3小时,随后冷却至室温过夜。反应混合物用EtOAc(200 mL)稀释,过滤除去盐,蒸发EtOAc后得到DMSO溶液。将该溶液用水稀释,形成沉淀。过滤收集沉淀,用水洗涤,真空干燥,得到1-叔丁基羰基-4-(5-硝基-2-吡啶基)哌嗪(16.1 g,93%)为浅橙色固体。1H NMR(400 MHz,CDCl3)δ ppm 1.47(s,9H),3.55(m,4H),3.75(m,4H),6.55(d,J = 9.3 Hz,1H),8.21(dd,J = 9.5,2.7 Hz,1H),9.03(d,J = 2.7 Hz,1H)。将1-叔丁基羰基-4-(5-硝基-2-吡啶基)哌嗪(16.0 g,51.9 mmol)溶于THF(400 mL)中,加入Raney镍(4 g),在50 psi氢气气氛下反应5小时。通过硅藻土过滤除去催化剂,真空蒸发溶剂,得到1-叔丁基羰基-4-(5-氨基-2-吡啶基)哌嗪(14.5 g,100%)。1H NMR(400 MHz,CDCl3)δ ppm 1.46(s,9H),3.31(m,6H),3.53(m,4H),6.56(d,J = 8.8 Hz,1H),6.98(dd,J = 8.8,2.9 Hz,1H),7.78(dd,J = 2.9,0.7 Hz,1H)。m/z 279.1(M + 1)。
参考文献:
[1] Patent: US2005/182078, 2005, A1. Location in patent: Page/Page column 9; 12
[2] Tetrahedron Letters, 2011, vol. 52, # 45, p. 5905 - 5909
[3] Patent: WO2004/9587, 2004, A1. Location in patent: Page 36
[4] Patent: EP2773638, 2015, B1. Location in patent: Paragraph 0753; 0754
[5] Patent: US2004/9988, 2004, A1. Location in patent: Page/Page column 10
