19477-73-7
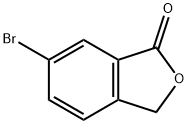 19477-73-7 结构式
19477-73-7 结构式
基本信息
6-溴苯酞
6-溴-3H-异苯并呋喃-1-酮
6-溴-2-苯并呋喃-1(3H)酮
6-溴苯肽 ( 粗品)(6-溴-2-苯并呋喃-1(3H)酮)
Roxadustat Impurity 12
6-bromo-1(3H)-isobenzofuanone
6-bromo-1H-isobenzofuran-1-one
6-bromo-2-benzofuran-1(3H)-one
6-BROMO-3 H-ISOBENZOFURAN-1-ONE
1(3H)-Isobenzofuranone, 6-broMo-
6-broMo-1,3-dihydro-2-benzofuran-1-one
物理化学性质
制备方法
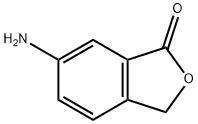
57319-65-0

19477-73-7
步骤c:6-溴异苯并呋喃-1(3H)-酮的合成 将NaNO2(2.2 g,0.040 mol)的水(22 mL)溶液逐滴加入至6-氨基异苯并呋喃-1(3H)-酮(5.0 g,0.030 mol)在HBr(70 mL,48%)的悬浮液中,保持反应温度在0℃。滴加完成后,继续搅拌反应混合物20分钟。随后,将反应液转移至预先冷却的CuBr(22 g,0.21 mol)在HBr(48%,23 mL)的溶液中。搅拌所得深棕色混合物20分钟后,用去离子水(200 mL)稀释,析出橙色沉淀。过滤收集沉淀,用饱和NaHCO3溶液洗涤,随后用乙酸乙酯(20 mL×3)萃取。合并有机相,用无水Na2SO4干燥,减压浓缩,得到6-溴异苯并呋喃-1(3H)-酮(5.4 g,产率84%)。 1H NMR(300 MHz,CDCl3)δ 8.05(d,J = 1.8 Hz,1H),7.80(dd,J = 8.1,1.8 Hz,1H),7.39(d,J = 8.1 Hz,1H),5.28(s,2H)。
参考文献:
[1] Patent: US2008/9524, 2008, A1. Location in patent: Page/Page column 410
[2] Patent: EP1362856, 2003, A1. Location in patent: Page/Page column 159
[3] Patent: US2015/231142, 2015, A1. Location in patent: Paragraph 1265
[4] Chemistry of Heterocyclic Compounds, 2010, vol. 46, # 2, p. 140 - 145
[5] Journal of Medicinal Chemistry, 2015, vol. 58, # 9, p. 3997 - 4015

