196603-96-0
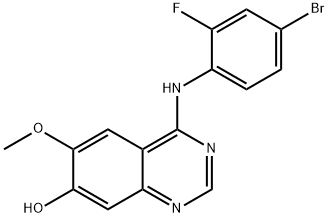 196603-96-0 结构式
196603-96-0 结构式
基本信息
4-(4-溴-2-氟苯胺基)-7-羟基-6-甲氧基喹唑啉
6-甲氧基-7-羟基-4-(2''-氟-4''-溴苯胺)喹唑啉
4-(4-bromo-2-fluoroanilino)-6-methoxy-1H-quinazolin-7-one
4-[(4-Bromo-2-fluorophenyl)amino]-6-methoxy-7-Quinazolinol
4-(4-Bromo-2-fluoroanilino)-7-hydroxy-6-methoxyquinazoline
7-Quinazolinol, 4-[(4-broMo-2-fluorophenyl)aMino]-6-Methoxy-
6-Methoxy-7-hydroxy-4-(2''-fluoro-4''-broMoaniline)quinazoline
4-(4-Bromo-2-fluoroanilino)-7-hydroxy-6-methoxyquinazoline ISO 9001:2015 REACH
物理化学性质
制备方法
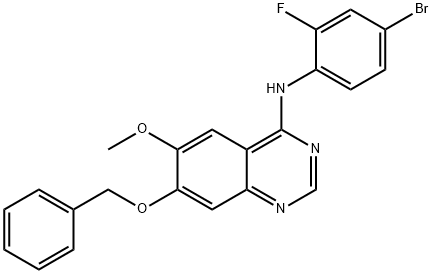
768350-54-5

196603-96-0
以7-(苄氧基)-N-(4-溴-2-氟苯基)-6-甲氧基喹唑啉-4-胺为原料合成4-(4-溴-2-氟苯胺基)-7-羟基-6-甲氧基喹唑啉的一般步骤:将中间体7-(苄氧基)-N-(4-溴-2-氟苯基)-6-甲氧基喹唑啉-4-胺(1.30g,2.86mmol)溶于三氟乙酸(15mL)中,将溶液加热回流1.5小时。反应完成后,将溶液冷却至室温并减压浓缩。向残余的棕色固体中加入甲醇(20mL),用浓氢氧化铵溶液调节pH至11。将混合物减压浓缩并在高真空下干燥,然后通过硅胶柱(20g)进行快速柱色谱纯化,洗脱剂为5-20%甲醇的二氯甲烷溶液。此步骤得到目标产物4-(4-溴-2-氟苯胺基)-7-羟基-6-甲氧基喹唑啉(1.03g,收率99%),为浅黄色固体。1H NMR(300MHz,DMSO-d6):δ 10.5(br s,1H),9.53(br s,1H),8.34(s,1H),7.81(s,1H),7.63(d,1H,J = 9.6 Hz),7.54(dd,1H,J = 8.4, 7.8 Hz),7.44(d,1H,J = 8.4 Hz),7.12(s,1H),3.95(s,3H)。13C NMR(75MHz,DMSO-d6):δ 156.77, 156.49, 152.92, 152.62, 148.62, 146.74, 129.40, 127.39, 126.46(d,J = 12Hz),119.23(d,J = 23.2Hz),117.4(d,J = 8.3Hz),109.96, 108.08, 102.23, 56.09。
参考文献:
[1] Patent: WO2005/97134, 2005, A2. Location in patent: Page/Page column 17-18; figure 2
[2] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 11, p. 3222 - 3226
[3] Patent: US2010/75916, 2010, A1. Location in patent: Page/Page column 16
[4] Patent: WO2005/13998, 2005, A1. Location in patent: Page/Page column 75-76
[5] Patent: WO2012/30160, 2012, A2. Location in patent: Page/Page column 52
