1977-07-7
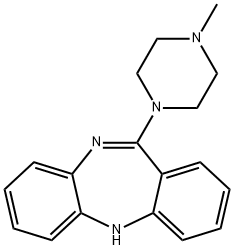 1977-07-7 结构式
1977-07-7 结构式
基本信息
11-(4-甲基-1-哌嗪基)-5H-二苯并[B,E][1,4]二氮杂卓
11-(4-甲基哌嗪-1-基)-5H-二苯并[B,E] [1,4]二氮杂卓
Dopamine serotonin antagonist-1
11-(4-Methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine
11-(4-methyl-1-piperazinyl)-5H-dibenzo(b,e)(1,4)diazepine
5H-Dibenzo[b,e][1,4]diazepine, 11-(4-methyl-1-piperazinyl)-
物理化学性质
制备方法
109-01-3
![5,11-二氢苯并[B][1,4]苯并二氮杂卓-6-酮](/CAS/GIF/5814-41-5.gif)
5814-41-5

1977-07-7
以N-甲基哌嗪和5,10-二氢-11H-二苯并[b,e][1,4]二氮杂卓-11-酮为原料合成11-(4-甲基哌嗪-1-基)-5H-二苯并[b,E][1,4]二氮杂卓的一般步骤:将5,10-二氢-11H-二苯并[b,e][1,4]二氮杂卓-11-酮(105 mg,0.5 mmol)置于50 mL圆底烧瓶中。依次加入N,N-二甲基苯胺(36.3 mg,0.3 mmol)。在130°C条件下,加入适量超干三氯氧磷和磁力搅拌子,在氮气保护下,将混合物在回流条件下搅拌12小时。反应完成后,蒸馏除去过量的三氯氧磷。随后,加入适量N-甲基哌嗪继续搅拌12小时,反应终止。通过薄层色谱(TLC)监测反应进程。反应混合物用乙酸乙酯(3×70 mL)萃取,依次用水洗涤。合并有机层,加入无水Na2SO4干燥。过滤后,浓缩残余物。通过柱色谱(洗脱剂:二氯甲烷/甲醇 = 25:1)纯化,得到110 mg目标产物11-(4-甲基哌嗪-1-基)-5H-二苯并[b,E][1,4]二氮杂卓,产率为75%。
参考文献:
[1] Patent: CN108586364, 2018, A. Location in patent: Paragraph 0150; 0151; 0152
常见问题列表
Ki: 6.3 nM (hM 3 Dq), 4.2 nM (hM 4 Di)
Deschloroclozapine has greater potencies for DREADDs than previous agonists in vitro. Deschloroclozapine is a potent agonist for hM
3
Dq with an EC
50
=0.13 nM. Deschloroclozapine is also a potent agonist for hM
4
Di with an EC
50
=0.081 nM.
Deschloroclozapine is a potent and selective agonist for hM
3
Dq and hM
4
Di, it does not display significant agonistic activity for any of the 318 tests wild-type GPCRs at <10 nM.
Deschloroclozapine (100 μg/kg; i.v.) exhibits good brain concentration profiles and biostability. Pharmacokinetic studies confirmed that Deschloroclozapine is rapidly accumulated in mouse brains and monkey CSF, while its metabolites are negligible.
Deschloroclozapine (1 μg/kg; i.p.) selectively and rapidly enhances neuronal activity via hM
3
Dq-DREADD in vivo, Deschloroclozapine can also be utilized for in vivo neuronal silencing by activating hM
4
Di, an inhibitory DREADD.
Deschloroclozapine (1-100 μg/kg; i.v.) selectively induces hM
3
Dq-mediated metabolic activity.
Deschloroclozapine (100 μg/kg; i.m.) selectively induces behavioral deficits in hM
4
Di-expressing monkeys.
| Animal Model: | Macaque monkey; 2.8-8.0 kg; age 3-10 years |
| Dosage: | 10, 100, 1000, 10000 μg/kg |
| Administration: | I.v. bolus injection |
| Result: | Required the dose for 50% occupancy (ED 50 ) for Deschloroclozapine was 25 μg/kg. |
| Animal Model: | Macaque monkey; 2.8-8.0 kg; age 3-10 years |
| Dosage: | 100 μg/kg (Pharmacokinetic Analysis) |
| Administration: | I.v. injection |
| Result: | Provided a sufficient concentration of Deschloroclozapine by a low systemic dose of Deschloroclozapine to be available for hM 4 Di-DREADD binding in vivo for at least for 2 h without the production of metabolites in monkeys. |
| Animal Model: | Wild-type C57BL/6j mice; male; age >12 weeks |
| Dosage: | 100 μg/kg (Pharmacokinetic Analysis) |
| Administration: | I.p. administration |
| Result: |
Diminished rapidly of Deschloroclozapine concentration and were undetectable at 2 h in either brain tissue or CSF.
The amount of the desmethyl metabolite C21 in CSF was negligible. |
| Animal Model: | HM 3 Dq monkeys and non-DREADD monkeys |
| Dosage: | 1, 3, 100 μg/kg (Pharmacokinetic Analysis) |
| Administration: | I.v. injection |
| Result: | Increased of FDG uptaking after Deschloroclozapine administration occurred exclusively at the hM 3 Dq-positive area. |
| Animal Model: | Monkeys received multiple injections of an AAV-vector carrying hM 4 Di genes |
| Dosage: | 100 μg/kg (Pharmacokinetic Analysis) |
| Administration: | I.m. administration |
| Result: | Enabled a rapidly and reversibly-induced behavioral change through activating muscarinic-based DREADDs without significant side effects. |