199328-31-9
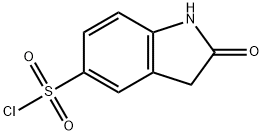 199328-31-9 结构式
199328-31-9 结构式
基本信息
2-氧代吲哚林-5-黄酰氯
2-氧代吲哚啉-5-磺酰氯
2-氧化二氢吲哚-5-磺酰氯
2-氧代-2,3-二氢-1H-吲哚-5-磺酰氯
2-OXOINDOLINE-5-SULP
Indolone-5-sulfonyl chloride
5-CHLOROSULPHONYL-2-OXINDOLE
5-chlorosulfonyl-2-indolinone
2-Oxindole-5-sulfonyl chloride
2-Oxindole-5-sulphonyl chloride
2-Oxindole-5-sulphonylchloride97%
2-Oxoindoline-5-sulphonyl chloride
2-Oxindole-5-sulphonyl chloride 97%
物理化学性质
制备方法
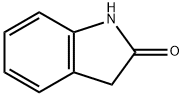
59-48-3

199328-31-9
以2-吲哚酮为原料合成2-氧代吲哚啉-5-磺酰氯的一般步骤:在搅拌下,将氯磺酸(27 mL,408 mmol)缓慢滴加到吲哚啉-2-酮(13.3 g,100 mmol)中,控制滴加速度以保持反应温度低于30℃。滴加完毕后,将反应混合物在室温下继续搅拌1.5小时,随后升温至68℃搅拌1小时。反应完成后,将混合物冷却至室温,并小心倒入水中。通过真空过滤收集析出的固体产物,用水洗涤,并在真空烘箱中干燥,得到2-氧代吲哚啉-5-磺酰氯(3.02 g,13.03 mmol,收率98%),该产物无需进一步纯化即可用于后续反应。产物的熔点为180-185℃。1H NMR (δ, ppm): 3.46 (s, 2H, CH2), 6.74 (d, 1H, J = 7.7 Hz, Ar), 7.42-7.46 (m, 2H, Ar), 10.48 (br s, 1H). 13C NMR (δ, ppm): 175.89, 143.68, 139.71, 124.55, 121.32, 107.39, 35.16. 元素分析(C8H6ClNO3S)计算值:C 41.48, H 2.61, N 6.05, S 13.84; 实测值:C 41.57, H 2.72, N 6.14, S 13.92。
参考文献:
[1] European Journal of Medicinal Chemistry, 2015, vol. 105, p. 274 - 288
[2] Journal of Antibiotics, 2018, vol. 71, # 10, p. 887 - 897
[3] Patent: CN104211632, 2016, B. Location in patent: Paragraph 0081; 0082
[4] Patent: WO2011/119777, 2011, A2. Location in patent: Page/Page column 26
[5] European Journal of Medicinal Chemistry, 2008, vol. 43, # 4, p. 755 - 762
