199522-66-2
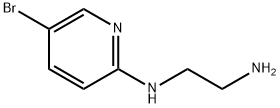 199522-66-2 结构式
199522-66-2 结构式
基本信息
N1-(5-Bromopyrid-2-yl)ethane-1,2-diamine
N1-(5-Bromo-2-pyridinyl)-1,2-ethanediamine
1,2-Ethanediamine, N1-(5-bromo-2-pyridinyl)-
物理化学性质
制备方法
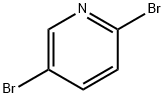
624-28-2
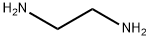
107-15-3

199522-66-2
以2,5-二溴吡啶和乙二胺为原料合成N1-(5-溴吡啶-2-基)乙烷-1,2-二胺的一般步骤如下:将1g(4.22mmol)2,5-二溴吡啶溶于10mL乙二胺中,所得溶液在100℃下搅拌反应15小时。反应完成后,将混合物冷却至室温,通过减压蒸馏除去过量的乙二胺。随后,向浓缩物中加入50mL二氯甲烷进行稀释,用30mL蒸馏水洗涤稀释后的溶液。将水相用50mL二氯甲烷萃取两次,以回收残余的有机产物。合并所有有机层,用无水硫酸钠干燥,然后减压浓缩,得到0.89g(4.12mmol)目标化合物N1-(5-溴吡啶-2-基)乙烷-1,2-二胺,为浅黄色液体,收率为98%。产物经1H NMR(400MHz,CDCl3)表征:δ8.10(d,J = 1.6Hz,1H),7.45(dd,J1 = 8.8Hz,J2 = 2.4Hz,1H),6.33(d,J = 8.8Hz,1H),4.95(brs,1H),3.34(q,J = 6 Hz,2H),2.94(t,J = 6 Hz,2H)。
参考文献:
[1] Patent: EP2692727, 2014, A2. Location in patent: Paragraph 0102-0103
[2] Patent: US2014/179691, 2014, A1. Location in patent: Paragraph 0112-0114
[3] Journal of Medicinal Chemistry, 1998, vol. 41, # 24, p. 4693 - 4705
[4] Patent: WO2017/106064, 2017, A1. Location in patent: Page/Page column 60