203519-88-4
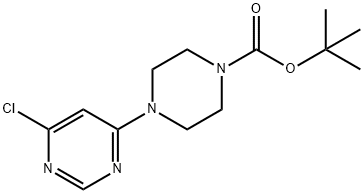 203519-88-4 结构式
203519-88-4 结构式
基本信息
4-(6-氯-4-嘧啶)-哌嗪-1-羧酸叔丁酯
4-(6-氯-嘧啶-4-基)-哌嗪-1-羧酸叔丁酯
1-Boc-4-(6-chloropyrimidin-4-yl)piperazine
ert-Butyl4-(6-chloropyrimidin-4-yl)piperazine-1-carboxylate
4-(6-Chloro-pyrimidin-4-yl)-piperazine-1-Carbocylic acid tert-butyl ester
4-(6-Chloro-pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester
1-Piperazinecarboxylic acid, 4-(6-chloro-4-pyrimidinyl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
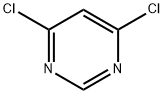
1193-21-1
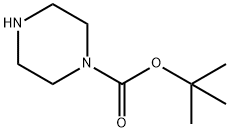
57260-71-6

203519-88-4
步骤1:4-(6-氯嘧啶-4-基)哌嗪-1-羧酸叔丁酯的合成 在微波反应管中,将4,6-二氯嘧啶(0.5g,3.36mmol)、N-BOC-哌嗪(0.69g,3.36mmol)和三乙胺(0.34g,3.36mmol)溶于乙腈(10mL)中。将反应混合物在120℃下微波加热2小时。反应完成后,将混合物用乙酸乙酯稀释,依次用饱和氯化铵溶液洗涤,无水硫酸镁干燥。过滤除去干燥剂,滤液经减压浓缩,得到目标化合物4-(6-氯嘧啶-4-基)哌嗪-1-羧酸叔丁酯(0.82g,收率91%)。 1H NMR(500MHz,CDCl3):δ 8.39(s,1H),6.50(s,1H),3.65(m,4H),3.52(m,4H),1.48(s,9H)。
参考文献:
[1] Patent: WO2012/69852, 2012, A1. Location in patent: Page/Page column 69
[2] Patent: CN106045918, 2016, A. Location in patent: Paragraph 0098; 0099
[3] Patent: US2009/163508, 2009, A1. Location in patent: Page/Page column 21
[4] Patent: WO2007/56023, 2007, A2. Location in patent: Page/Page column 57
[5] Patent: US2009/163508, 2009, A1. Location in patent: Page/Page column 36
