203866-16-4
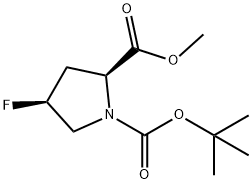 203866-16-4 结构式
203866-16-4 结构式
基本信息
N-叔丁氧羰基-顺式-4-氟-L-脯氨酸甲酯
(2S,4S)-N-BOC-4-氟吡咯烷-2-羧酸甲酯
(2S,4S)-N-BOC-顺式-4-氟-L-脯氨酸甲酯
(2S,4S)-1-叔丁基-2-甲基-4-氟吡咯烷-1,2-二羧酸酯
N-t-BOC-cis-4-fluoro-L-proline methyl ester
(2S,4S)-N-Boc-cis-4-fluoro-L-proline methyl ester
N-Boc-cis-4-Fluoro-L-proline methyl ester USP/EP/BP
Ethyl (2S,4S)-1-Boc-4-fluoropyrrolidine-2-carboxylate
N-(tert-Butoxycarbonyl)-(2S,4S)-4-fluoroproline Methyl ester
(2S)-1-tert-butyl 2-methyl 4-fluoropyrrolidine-1,2-dicarboxylate
1-tert-butyl 2-Methyl (2S,4S)-4-fluoro-1,2-pyrrolidinedicarboxylate
1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate
(2S,4S)-4-Fluoro-1,2-pyrrolidinedicarboxylic acid 1-(tert-butyl) 2-methyl ester
物理化学性质
制备方法
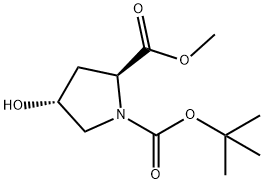
74844-91-0

203866-16-4
以N-Boc-反式-4-羟基-L-脯氨酸甲酯为原料合成N-叔丁氧羰基-顺式-4-氟-L-脯氨酸甲酯的一般步骤:在-78℃下,向N-Boc-反式-4-羟基-L-脯氨酸甲酯(11g,44.84mmol)的二氯甲烷(200mL)溶液中逐滴加入二乙氨基三氟化硫(Et2NSF3,8.85mL,67.3mmol)。滴加完毕后,将反应混合物在-78℃下继续搅拌2小时,随后缓慢升温至室温并搅拌19小时。反应完成后,用氯化铵水溶液(100mL)淬灭反应。用二氯甲烷(100mL×3)萃取反应混合物。合并有机层,用无水硫酸钠干燥,减压浓缩。残余物通过硅胶柱色谱纯化,洗脱剂为石油醚/乙酸乙酯(v/v=20/1),得到N-叔丁氧羰基-顺式-4-氟-L-脯氨酸甲酯,为浅黄色固体(5.0g,收率70%)。该化合物的结构通过以下光谱数据确认:质谱(ESI正离子模式)m/z:248.26 [M+H]+;核磁共振氢谱(400MHz,CDCl3)δ(ppm):5.26和5.13(双峰,1H),4.55-4.41(多重峰,1H),3.88-3.74(多重峰,1H),3.73(单峰,3H),3.64-3.58(多重峰,1H),2.52-2.44(多重峰,1H),2.40-2.32(多重峰,1H),1.42-1.47(双峰,9H,J=20Hz)。
参考文献:
[1] Patent: WO2014/19344, 2014, A1. Location in patent: Paragraph 00434
[2] Patent: WO2014/82380, 2014, A1. Location in patent: Paragraph 00403; 00404
[3] Patent: WO2014/82379, 2014, A1. Location in patent: Page/Page column 144; 145
[4] Patent: US2015/79028, 2015, A1. Location in patent: Paragraph 1061; 1062; 1063; 1064; 1065
[5] Patent: CN103880823, 2017, B. Location in patent: Paragraph 1003-1005