204203-16-7
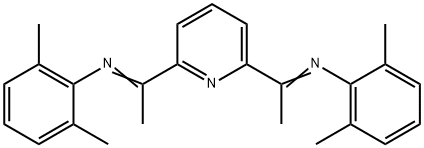 204203-16-7 结构式
204203-16-7 结构式
基本信息
1,1'-(Pyridine-2,6-diyl)bis(N-(2,6-dimethylphenyl)ethan-1-imine)
1,1'-(Pyridine-2,6-diyl)bis(N-(2,6-dimethylphenyl)ethan-1-imine)
Benzenamine, N,N'-(2,6-pyridinediyldiethylidyne)bis[2,6-dimethyl-
2,6-Bis[1-[(2,6-diMethylphenyl)iMino]ethyl]pyridine ISO 9001:2015 REACH
N,N'-(Pyridine-2,6-diylbis(ethan-1-yl-1-ylidene))bis(2,6-dimethylaniline)
N,N'-(Pyridine-2,6-diylbis(ethan-1-yl-1-ylidene))bis(2,6-dimethylaniline)
制备方法
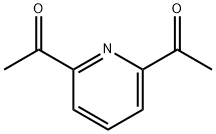
1129-30-2
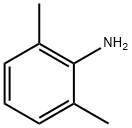
87-62-7
![2,6-双[1-[(2,6-二甲基苯基)亚氨基]乙基]吡啶](/CAS/GIF/204203-16-7.gif)
204203-16-7
1. 2,6-二乙酰基吡啶双(2,6-二甲基苯基亚胺)(2,6-Me2PDI)的合成 在250 mL圆底烧瓶中,依次加入4.0 g 2,6-二乙酰基吡啶、6.09 g(2.05当量)2,6-二甲基苯胺和100 mL甲醇。随后加入催化量的对甲苯磺酸,将反应混合物置于配备Dean-Stark分水器的回流装置中,分水器中预先加入硫酸钾。反应混合物回流过夜后,冷却至室温,并通过旋转蒸发仪将甲醇体积浓缩至约起始体积的一半。将浓缩后的混合物冷却至-35℃,过滤收集析出的固体,得到4.5 g(产率50%)目标产物2,6-Me2PDI。滤液可进一步浓缩并冷却以获得更多产物。产物结构经1H NMR(苯-d6,200 MHz)确认:δ= 8.50(d,2H,吡啶间位氢),7.28(t,1H,吡啶对位氢),7.08(d,4H,芳环间位氢),6.99(t,2H,芳环对位氢),2.17(s,6H,亚甲基氢),2.05(s,12H,芳环甲基氢)。
参考文献:
[1] Dalton Transactions, 2017, vol. 46, # 23, p. 7408 - 7411
[2] Chemistry - A European Journal, 2014, vol. 20, # 16, p. 4754 - 4761
[3] Dalton Transactions, 2013, vol. 42, # 24, p. 8802 - 8807
[4] Journal of Molecular Catalysis A: Chemical, 2011, vol. 340, # 1-2, p. 83 - 88
[5] Patent: WO2011/6044, 2011, A2. Location in patent: Page/Page column 19-20