204905-77-1
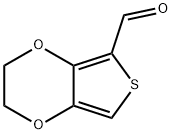 204905-77-1 结构式
204905-77-1 结构式
基本信息
3,4-乙烯二氧噻吩-2-甲醛
3,4-亚乙二氧基噻吩-2-甲醛
2-(3,4-乙烯基双氧噻吩)甲醛
2,3-二氢噻吩并[3,4-B][1,4]二恶烷-5-甲醛
2,3-二氢噻吩并[3,4-B][1,4]二恶烷-5-甲醛
2,3-二氢噻吩并[3,4-B][1,4]二噁英-5-甲醛
2,3-二氢噻吩并[3,4-B][1,4]二氧杂环己烯-5-甲醛
2-(3,4-乙烯基双氧噻吩)甲醛(3,4-乙撑基二氧甲醛噻吩 C7H6O3S)
2-Formyl-3,4-ethylenedioxythiophene
3,4-Ethylenedioxothiophene-2-carbaldehyde
3,4-Ethylenedioxythiophene-2-carbaldehyde
3,4-Ethylenedioxythiophene-2-carboxaldehyde
2,3-dihydrothieno[3,4-b][1,4]dioxine-5-carbaL
2,3-dihydrothieno[3,4-b][1,4]dioxine-5-carbaldehy
2,3-Dihydrothieno[3,4-b]-1,4-dioxin-5-carbaldehyde
2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXINE-5-CARBALDEHYDE
2,3-dihydro-Thieno[3,4-b]-1,4-dioxin-5-carboxaldehyde
物理化学性质
制备方法
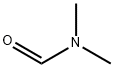
68-12-2
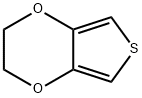
126213-50-1

204905-77-1
以N,N-二甲基甲酰胺(DMF)和3,4-乙烯二氧噻吩(EDOT)为原料合成2-(3,4-乙烯基双氧噻吩)甲醛的一般步骤:在0℃条件下,向含有EDOT(15.0g, 0.1mol)和DMF(11.0g, 0.15mol)的混合溶液中缓慢滴加溶解于无水二氯甲烷(80mL)中的三光气(BTC, 11.9g, 0.04mol)。将反应混合物升温至35℃并持续搅拌1小时,随后冷却至室温并倒入冰水(250mL)中。用10%氢氧化钠溶液调节水相的pH至7-8,分离有机相。水相用二氯甲烷萃取三次。合并所有有机相,用无水硫酸镁干燥。减压蒸馏去除溶剂后,粗产物通过95%乙醇重结晶,得到白色针状晶体产物(15.5g, 收率86.3%)。产物经1H NMR(400MHz)表征:δ/ppm: 9.90(s, 1H), 6.79(s, 1H), 4.38-4.25(m, 4H)。
参考文献:
[1] Advanced Functional Materials, 2011, vol. 21, # 4, p. 634 - 643
[2] Tetrahedron, 1999, vol. 55, # 40, p. 11745 - 11754
[3] Journal of the American Chemical Society, 2004, vol. 126, # 50, p. 16440 - 16450
[4] Physical Chemistry Chemical Physics, 2015, vol. 17, # 8, p. 5776 - 5784
[5] Russian Journal of Applied Chemistry, 2010, vol. 83, # 8, p. 1435 - 1439
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | E1150 | 3,4-乙烯二氧噻吩-2-甲醛 3,4-Ethylenedioxythiophene-2-carboxaldehyde | 204905-77-1 | 1g | 250元 |
| 2025/12/22 | XW0220490577103 | 3,4-亚乙二氧基噻吩-2-甲醛 3,4-Ethylenedioxythiophene-2-carbaldehyde | 204905-77-1 | 5g | 97元 |
| 2025/12/22 | E1150 | 3,4-乙烯二氧噻吩-2-甲醛 3,4-Ethylenedioxythiophene-2-carboxaldehyde | 204905-77-1 | 5g | 670元 |
