20781-23-1
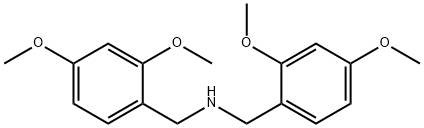 20781-23-1 结构式
20781-23-1 结构式
基本信息
双(2,4-二甲氧基苯基)胺
二(2,4-而甲氧基苯甲基)胺
Bis(2,4-dimethoxybenzyl)
Bis(2,4-dimethoxybenyl)amine
BIS(2,4-DIMETHOXYBENZYL)AMINE
Bis(2,4-dimenthoxybenzyl)amine
2,2',4,4'TETRAMETHOXYDIBENZYLAMINE
bis[(2,4-diMethoxyphenyl)Methyl]aMine
Benzenemethanamine, N-(2,4-dimethoxyphenyl)methyl-2,4-dimethoxy-
1-(2,4-DIMETHOXYPHENYL)-N-[(2,4-DIMETHOXYPHENYL)METHYL]METHANAMINE
物理化学性质
制备方法
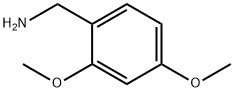
20781-20-8
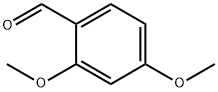
613-45-6

20781-23-1
在室温条件下,将2,4-二甲氧基苯甲醛(Sigma Aldrich; 1.1g,6.61mmol)溶解于20mL四氢呋喃(THF)中。向此溶液中逐滴加入2,4-二甲氧基苄胺(Sigma Aldrich; 1.5mL,9.98mmol),随后加入三乙酰氧基硼氢化钠(Sigma Aldrich; 17g; 80.4mmol)。反应混合物逐渐变得混浊,于室温下搅拌反应过夜。反应完成后,用饱和碳酸氢钠(NaHCO3)溶液稀释反应混合物,随后用乙酸乙酯(EtOAc)进行萃取。合并有机相,用无水硫酸镁(MgSO4)干燥,过滤后减压浓缩。所得粗产物通过硅胶柱色谱法纯化(使用40g ISCO柱,洗脱梯度为0至100%乙酸乙酯/己烷),得到双(2,4-二甲氧基苄基)胺(2.08g,6.55mmol,产率99%,纯度96%)。
参考文献:
[1] Patent: WO2017/147410, 2017, A1. Location in patent: Page/Page column 1832
[2] Tetrahedron Letters, 2010, vol. 51, # 28, p. 3645 - 3648
[3] Chemische Berichte, 1968, vol. 101, # 10, p. 3623 - 3641
[4] Patent: US2013/131050, 2013, A1. Location in patent: Paragraph 0570; 0571
[5] Patent: WO2018/97945, 2018, A1. Location in patent: Paragraph 0395
