209984-30-5
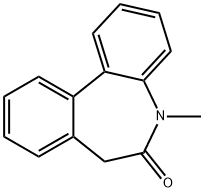 209984-30-5 结构式
209984-30-5 结构式
基本信息
5,7-二氢-5-甲基-6H-二苯并[B,D]氮杂卓-6-酮
5-methyl-7H-benzo[d][1]benzazepin-6-one
N-methyl-5H-dibenzo[b,d]azepin-6(7H)-one
5,7-Dihydro-5-methyl-6H-dibenz[b,d]azepin-6-one
5-methyl-5,7-dihydro-6H-dibenzo[b,d]azepin-6-one
6H-DIBENZ[B,D]AZEPIN-6-ONE, 5,7-DIHYDRO-5-METHYL-
8-methyl-8-azatricyclo[9.4.0.0^{2,7}]pentadeca-1(15),2(7),3,5,11,13-hexaen-9-one
5-methyl-5H-dibenzo[b,d]azepin-6(7H)-one 5,7-Dihydro-5-methyl-6H-dibenz[b,d]azepin-6-one
物理化学性质
制备方法
![5H-二苯并[B,D]氮杂卓-6(7H)-酮](/CAS2/GIF/20011-90-9.gif)
20011-90-9

74-88-4
![5,7-二氢-5-甲基-6H-二苯并[B,D]氮杂卓-6-酮](/CAS/GIF/209984-30-5.gif)
209984-30-5
步骤A-合成7-甲基-5,7-二氢-6H-二苯并[b,d]氮杂卓-6-酮:在干燥的圆底烧瓶中加入氢化钠(0.295 g,7.46 mmol)和9.0 mL无水DMF,搅拌至完全溶解。随后加入5,7-二氢-6H-二苯并[b,d]氮杂卓-6-酮(1.3 g,6.22 mmol),反应混合物在60℃下搅拌1小时。然后,缓慢滴加碘甲烷(1.16 mL,18.6 mmol),避光条件下继续搅拌17小时。反应完成后,将混合物冷却至室温,用二氯甲烷和水的混合液稀释,依次用NaHSO4溶液和水洗涤,有机相用无水Na2SO4干燥。减压浓缩后,通过快速柱色谱(硅胶,氯仿作为洗脱剂)纯化,得到0.885 g(产率63%)目标化合物5-甲基-5H-二苯并[b,d]氮杂卓-6(7H)-酮,为无色固体。核磁共振氢谱(1H-NMR, CDCl3)数据:δ= 7.62(d, 2H),7.26-7.47(m, 6H),3.51(m, 2H),3.32(s, 3H)。元素分析:C15H13NO(分子量=223.27);质谱(MH+)223。计算值:C, 80.69;H, 5.87;N, 6.27。实测值:C, 80.11;H, 5.95;N, 6.23。
参考文献:
[1] Organic Letters, 2008, vol. 10, # 21, p. 4871 - 4874
[2] Journal of Organic Chemistry, 2010, vol. 75, # 17, p. 5984 - 5993
[3] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 22, p. 6392 - 6395
[4] Patent: EP951466, 2009, B1. Location in patent: Page/Page column 72
[5] Patent: US6958330, 2005, B1. Location in patent: Page/Page column 38-39