2140-67-2
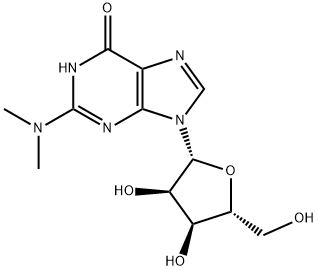 2140-67-2 结构式
2140-67-2 结构式
基本信息
N2,N2-二甲基鸟苷
M(2/2) GUO
2-(DIMETHYLAMINO)GUA
2,2-DiMethylguanosine
N,N-Dimethylguanosine
N2,N2-DIMETHYLGUANOSINE
2-(DIMETHYLAMINO)GUANOSINE
2-Dimethylamino-D-guanosine
N(sup 2),N(sup 2)-dimethylguanosine
2-DiMethylaMino-6-oxypurine Riboside
物理化学性质
制备方法
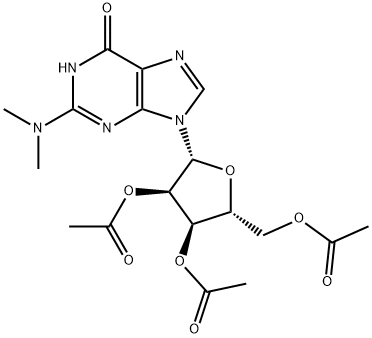
73196-87-9

2140-67-2
将化合物1(CAS: 73196-87-9,1.5 g,3.4 mmol)溶解于吡啶(2 mL)中,随后加入氨水。反应混合物在室温下搅拌3小时,通过薄层色谱(TLC)监测反应进度,直至显示完全脱保护。反应完成后,将溶剂在减压下蒸发至干。粗产物通过从水中重结晶进行纯化,得到目标产物9-((2R,3R,4S,5R)-3,4-二羟基-5-(羟甲基)四氢呋喃-2-基)-2-(二甲氨基)-1,9-二氢-6H-嘌呤-6-酮,产量0.68 g,收率65%。产物的结构通过1H NMR、13C NMR和质谱(ES-TOF)进行确认。1H NMR (DMSO-d6) δ: 3.06 (6H, s, NMe2), 3.48-3.63 (2H, m, 5'-CH2), 3.86 (1H, q, J = 4 Hz, H4'), 4.11 (1H, q, J = 4 Hz, H3'), 4.50 (1H, q, J = 4 Hz, H2'), 4.91 (1H, t, J = 5.4 Hz, OH5'), 5.15 (1H, d, J = 4.8 Hz, OH3'), 5.38 (1H, d, J = 6.1 Hz, OH2'), 5.71 (1H, d, J = 5.8 Hz, H1'), 5.92 (1H, s, H8), 10.69 (1H, bs, NH); 13C NMR δ: 38.1 (NMe2), 61.8 (C5'), 70.7 (C3'), 73.7 (C2'), 85.4 (C4'), 87.0 (C1'), 100.0, 116.4, 136.8 (C8), 151.1, 153.3, 157.7; MS (ES-TOF) m/z: 计算值 312.13,实测值 312.54。
参考文献:
[1] Journal of Organic Chemistry, 1991, vol. 56, # 3, p. 1224 - 1227
[2] Journal of Organic Chemistry, 1996, vol. 61, # 13, p. 4412 - 4422
[3] Patent: US9067964, 2015, B2. Location in patent: Page/Page column 15