214360-58-4
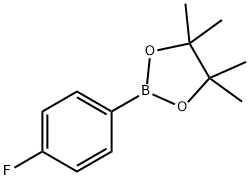 214360-58-4 结构式
214360-58-4 结构式
基本信息
4-氟苯硼酸频哪醇酯
4-氟苯硼酸频呐醇酯
4-氟苯硼酸频呢醇酯
4-氟苯基硼酸频那醇酯
4-氟苯基硼酸,频哪醇酯
4-氟苯硼酸频哪醇酯 1G
4-(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)氟苯
4-(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)氟苯/4-氟苯硼酸频哪醇酯
SWZn-214360-58-4
4-FLUOROPHENYLBORONIC ACID, PINACOL ESTER
4-Fluorobenzeneboronic acid, pinacol ester
4-Fluorobenzeneboronic acid, pinacol ester 97%
2-(4-Fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
1,3,2-Dioxaborolane,2-(4-fluorophenyl)-4,4,5,5-tetraMethyl-
4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)FLUOROBENZENE
1-Fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene
4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-FLUOROBENZENE 90%
物理化学性质
安全数据
制备方法

73183-34-3
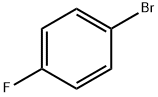
460-00-4

214360-58-4
一般步骤: 1. 搅拌反应:在氩气保护下,将乙酸钾(KOAc,0.073 g,0.09 mmol)和1,1'-双(二苯基膦基)二茂铁-二氯化钯(II)-二氯甲烷络合物(PdCl2(dppf)·CH2Cl2,0.613 g,3.5 mmol)加入至含有对溴氟苯(1b,R = F,0.613 g,3.5 mmol)和双(频哪醇合)二硼(2,1.161 g,4.5 mmol)的二甲基亚砜(DMSO,1.601 g)溶液中。将反应混合物在80℃下搅拌1.5小时。 2. 萃取:反应完成后,将反应液冷却至室温,加入冰水淬灭反应,随后用乙酸乙酯(EtOAc,80 mL × 2)萃取有机层。 3. 干燥与浓缩:合并有机层,用无水硫酸镁(MgSO4)干燥,过滤后在减压下浓缩,得到粗产物。 4. 纯化:粗产物通过柱色谱法(硅胶,洗脱剂:己烷/EtOAc = 9:1)纯化,得到4-氟苯硼酸频哪醇酯(3b,R = F),收率为93%。
参考文献:
[1] Patent: JP5966165, 2016, B2. Location in patent: Paragraph 0056-0062
[2] Journal of the American Chemical Society, 2016, vol. 138, # 9, p. 2985 - 2988
[3] Advanced Synthesis and Catalysis, 2016, vol. 358, # 6, p. 977 - 983
[4] Journal of Fluorine Chemistry, 2015, vol. 178, p. 195 - 201
[5] ACS Catalysis, 2018, vol. 8, # 5, p. 4049 - 4054
