218594-15-1
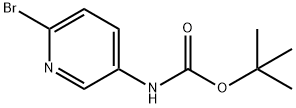 218594-15-1 结构式
218594-15-1 结构式
基本信息
2-溴-5-BOC-氨基砒啶
2-溴-5-(叔丁氧羰基氨基)吡啶
(6-溴吡啶-3-基)氨基甲酸叔丁酯
N-(6-溴吡啶-3-基)氨基甲酸叔丁酯
tert-butyl 6-bromopyridin-3-ylcarbamate
5-Amino-2-bromopyridine, 5-BOC protected
2-BROMO-5-AMINOPYRIDINE, N-BOC PROTECTED
5-Amino-2-bromopyridine,5-BOCprotected95%
tert-butyl N-(6-broMopyridin-3-yl)carbaMate
2-Bromo-5-aminopyridine, N-BOC protected 95%
2-BROMO-5-(TERT-BUTOXYCARBONYLAMINO)PYRIDINE
5-Amino-2-bromopyridine, 5-BOC protected 95%
(6-BROMO-PYRIDIN-3-YL)-CARBAMIC ACID TERT-BUTYL ESTER
物理化学性质
制备方法
13534-97-9

24424-99-5

218594-15-1
向6-溴吡啶-3-胺(5.4 g,28.9 mmol)的二氯甲烷(200 mL)溶液中依次加入三乙胺(4.4 g,43.3 mmol)和二碳酸二叔丁酯(7.6 g,34.7 mmol)。将反应混合物在室温下搅拌4小时,反应完成后,用水(300 mL)淬灭反应。分离有机层,并用无水硫酸钠干燥。过滤除去干燥剂,滤液经减压浓缩后,所得残余物通过硅胶柱色谱纯化,洗脱剂为2-10%乙酸乙酯的石油醚溶液,得到目标产物(6-溴吡啶-3-基)氨基甲酸叔丁酯,为无色固体。质谱(ESI, m/z):273.1, 275.1 [M + 1]+;1H NMR(300 MHz, DMSO-d6)δ 9.72(s, 1H), 8.45(d, J = 1.8 Hz, 1H), 7.83(d, J = 5.4 Hz, 1H), 7.53(d, J = 5.7 Hz, 1H), 1.48(s, 9H)。
参考文献:
[1] Patent: WO2015/188368, 2015, A1. Location in patent: Page/Page column 114
[2] Journal of Medicinal Chemistry, 2010, vol. 53, # 24, p. 8709 - 8715
[3] Chemistry - A European Journal, 2010, vol. 16, # 30, p. 9056 - 9067
[4] Patent: WO2017/207813, 2017, A1. Location in patent: Page/Page column 126
[5] Patent: WO2006/92599, 2006, A2. Location in patent: Page/Page column 58-59
