223131-01-9
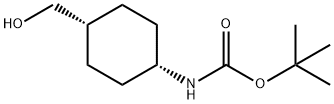 223131-01-9 结构式
223131-01-9 结构式
基本信息
顺式-4-(羟甲基)环己基氨基甲酸叔丁酯
顺式-4-(羟基甲基)环己基]氨基甲酸叔丁酯
顺-1-(BOC-氨基)-4-(羟甲基)环己烷
顺式-1-(Boc-氨基)-4-(羟甲基)环己烷
顺式-N-[4-(羟甲基)环己基]氨基甲酸叔丁酯
CIS-1-(BOC-氨基)-4-(羟甲基)环己烷
顺-1-(Boc-氨基)-4-(羟甲基)环己烷 223131-01-9
TERT-BUTYL CIS-4-(HYDROXYMETHYL)CYCLOHEXYLCABAMATE
tert-Butyl N-[4-(hydroxymethyl)cyclohexyl]carbamate
cis-1-(Boc-aMino)-4-(hydroxyMethyl)cyclohexane, 97%
TERT-BUTYL CIS-(4-HYDROXYMETHYL)CYCLOHEXYLCARBAMATE
Cis tert-butyl -4-(hydroxymethyl)cyclohexyl)carbamate
tert-butyl N-[cis-4-(hydroxymethyl)cyclohexyl]carbamate
tert-butyl (1s,4s)-4-(hydroxymethyl)cyclohexylcarbamate
tert-butyl cis-N-[4-(hydroxymethyl)cyclohexyl]carbamate
Carbamic acid, N-[cis-4-(hydroxymethyl)cyclohexyl]-, 1,1-dimethylethyl ester
制备方法

24424-99-5
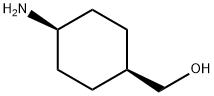
30134-98-6

223131-01-9
以二碳酸二叔丁酯和(1r,4r)-4-氨基环己基甲醇为原料合成顺-1-(Boc-氨基)-4-(羟甲基)环己烷的一般步骤:在室温下,将二碳酸二叔丁酯(3.04 g,13.9 mmol)缓慢加入至(1r,4r)-4-氨基环己基甲醇(1.50 g,11.6 mmol)的四氢呋喃(20 mL)搅拌溶液中。反应混合物在室温下搅拌过夜。随后,通过旋转蒸发去除溶剂,将残余物在乙酸乙酯和水之间分配。分离有机相,依次用水和饱和食盐水洗涤,无水硫酸镁干燥后,再次旋转蒸发去除溶剂。所得残余物用己烷处理,形成的悬浮液经过滤后,得到白色固体顺-1-(Boc-氨基)-4-(羟甲基)环己烷(2.11 g,收率79%)。产物经低分辨质谱(LRMS)检测,显示m/z为228(M-H)+。1H NMR(DMSO-d6)δ:0.84-0.95(m,2H),1.05-1.18(m,2H),1.20-1.29(m,2H),1.40(s,9H),1.71-1.80(m,3H),3.14(m,1H),3.21(t,2H),4.41(t,1H),6.73(d,1H)。
参考文献:
[1] Patent: EP2554544, 2013, A1. Location in patent: Paragraph 0161
[2] Journal of Labelled Compounds and Radiopharmaceuticals, 1999, vol. 42, # SUPPL. 1, p. S309-S310,S311
[3] Journal of Labelled Compounds and Radiopharmaceuticals, 1999, vol. 42, # 3, p. 293 - 307
[4] Patent: US2015/31674, 2015, A1. Location in patent: Paragraph 0274
