22766-68-3
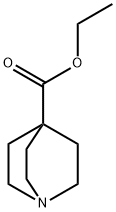 22766-68-3 结构式
22766-68-3 结构式
基本信息
奎宁环-4-羧酸乙酯
乙基奎宁环-4-羧酸酯
Quinuclidine-4-carboxylic acid ethyl ester
ethyl-1-azabicyclo[2.2.2]octane-4-carboxylate
azabicyclo[2.2.2]octane-4-carboxylic acid ethyl ester
1-Azabicyclo[2.2.2]octane-4-carboxylic acid ethyl ester
Diphenyl(quinuclidin-4-yl)methanolEthyl quinuclidine-4-carboxylate
物理化学性质
制备方法
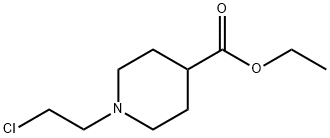
869112-14-1

22766-68-3
一般步骤:在氮气保护下,将1-(2-氯乙基)哌啶-4-甲酸乙酯(II)(5.0g,22.76mmol)溶于四氢呋喃(THF,147.0mL)中,并将溶液冷却至-50℃。在-50℃下,于25分钟内缓慢加入LDA(2.0M在庚烷/THF/乙苯中的溶液,17.0mL,34.0mmol)。反应混合物在16小时内逐渐升温至室温。反应完成后,用饱和K2CO3水溶液(122.0mL)淬灭反应,并用乙醚(3×120.0mL)萃取。合并有机层,用MgSO4干燥,过滤后减压浓缩。所得橙色液体与二氯甲烷共蒸发三次,以除去过量乙苯,最终得到橙色油状产物(4.15g,收率99.4%)。产物为1-氮杂双环[2.2.2]辛烷-4-羧酸乙酯(III),其1H-NMR(300MHz,CDCl3)数据如下:δ4.10(t,J=5.23Hz,2H),2.90-2.85(m,6H),1.71-1.66(m,6H),1.22(t,J=4.0Hz,3H)。
参考文献:
[1] Patent: WO2018/87561, 2018, A1. Location in patent: Page/Page column 13
[2] Patent: US2007/185155, 2007, A1. Location in patent: Page/Page column 9
[3] Journal of Medicinal Chemistry, 2009, vol. 52, # 8, p. 2493 - 2505
[4] Patent: WO2005/112644, 2005, A2. Location in patent: Page/Page column 13
[5] Angewandte Chemie - International Edition, 2017, vol. 56, # 4, p. 1152 - 1157
