22900-83-0
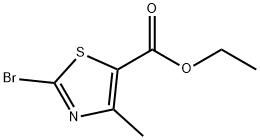 22900-83-0 结构式
22900-83-0 结构式
基本信息
2-溴-4-甲基噻唑-5-甲酸乙酯
2-溴-4-甲基-1,3-噻唑-5-甲酸乙酯
ETHYL 2-BROMO-4-METHYLTHIAZOLE-5-CARBOX&
ETHYL 2-BROMO-4-METHYLTHIAZOLE-5-CARBOXYLATE
Ethyl 2-Bromo-4-methyl-5-thiazolecarboxylate
Ethyl 2-broMo-4-Methylthiazole-5-carboxylate 97%
Ethyl 2-Bromo-4-methylthiazole-5-carboxylate >
Ethyl 2-bromop4-methyl-1,3-thiazole-5-carboxylate
ETHYL 2-BROMO-4-METHYL-1,3-THIAZOLE-5-CARBOXYLATE
2-Bromo-4-methyl-5-thiazolecarboxylic acid ethyl ester
2-bromo-4-methyl-thiazole-5-carboxylic acid ethyl ester
物理化学性质
安全数据
Skin Irrit. 2
Skin Sens. 1
STOT SE 3
制备方法
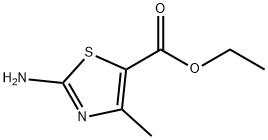
7210-76-6

22900-83-0
以2-氨基-4-甲基噻唑-5-羧酸乙酯为原料合成2-溴-4-甲基-1,3-噻唑-5-甲酸乙酯的一般步骤:首先,将2-甲基-1-硝基-丙基-丙烯(28.2 mL,2.1当量)溶解于乙腈(700 mL)中,并将溶液冷却至0°C。随后,缓慢滴加溴-三甲基-硅烷(32 mL,2.1当量)。将反应混合物维持在0°C。接着,加入2-氨基-4-甲基-噻唑-5-羧酸乙酯(18.6 g,0.1 mol)的乙腈/乙酸乙酯(75/25)溶液。滴加完毕后,继续在0°C下保持反应混合物。将混合物升温至室温并搅拌24小时。反应完成后,蒸发去除溶剂,加入水。用乙酸乙酯萃取产物,用无水硫酸钠干燥有机相,过滤并浓缩。最后,通过快速色谱法纯化,使用二氯甲烷作为洗脱液,得到2-溴-4-甲基-1,3-噻唑-5-甲酸乙酯,为黄色固体(21.74 g,87 mmol,收率87%);GC/MS分析:[M+] C7H8BrNO2S 250。
参考文献:
[1] Patent: WO2004/6923, 2004, A1. Location in patent: Page/Page column 38
[2] Patent: WO2004/6924, 2004, A1. Location in patent: Page/Page column 31-32
[3] Patent: WO2004/7493, 2004, A1. Location in patent: Page 24-25
[4] Patent: EP2518054, 2012, A1. Location in patent: Page/Page column 53
[5] Patent: WO2008/47109, 2008, A1. Location in patent: Page/Page column 38

