23361-28-6
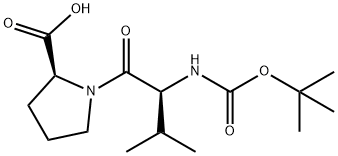 23361-28-6 结构式
23361-28-6 结构式
基本信息
N-[(1,1-二甲基乙氧基)羰基]-L-缬氨酰-L-脯氨酸
BOC-VAL-PRO-OH
-3-methylbutanoyl)
Boc-L-Val-L-Pro-OH
BOC-L-VALYL PROLINE
N-BOC-L-VALYL-L-PROLINE
(Tert-Butoxy)Carbonyl Val-Pro-OH
N-tert-Butoxycarbonyl-L-valyl-L-proline
N-(tert-butyloxycarbonyl)-L-valyl-L-proline
N-[(1,1-Dimethylethoxy)carbonyl]-L-valyl-L-proline
物理化学性质
制备方法
![L-Proline, N-[(1,1-dimethylethoxy)carbonyl]-L-valyl-, methyl ester](/CAS/20180808/GIF/38017-75-3.gif)
38017-75-3

23361-28-6
以化合物(CAS: 38017-75-3)为原料,采用改进的文献方法,通过碱水解反应制备N-[(1,1-二甲基乙氧基)羰基]-L-缬氨酰-L-脯氨酸(化合物2)。具体步骤如下:将化合物1(1.372 g,4.18 mmol)溶解于四氢呋喃(THF)和水的混合溶剂(体积比为3:1,总体积为80 mL,其中THF 60 mL,水20 mL)中。随后,加入氢氧化锂一水合物(LiOH·H2O,0.878 g,20.89 mmol),并将反应混合物在室温下搅拌2小时。反应完成后,用0.5 M盐酸(50 mL)酸化以淬灭反应,随后用乙酸乙酯(EtOAc,4×60 mL)进行萃取。合并有机相,用饱和食盐水(1×100 mL)洗涤,无水硫酸镁(MgSO4)干燥。减压蒸发除去溶剂,得到白色固体产物(1.186 g,收率90%)。产物的结构通过1H NMR、13C NMR和ESI-MS进行确证:1H NMR (400 MHz, CDCl3) δ 9.37 (br s, 1H, COOH), 5.46 (d, J = 9.4 Hz, 1H, NH), 4.55 (dd, J = 8.1, 4.8 Hz, 1H, Pro-αH), 4.26 (dd, J = 9.4, 6.8 Hz, 1H, Val-αH), 3.83-3.59 (m, 2H, Pro-δH), 2.25-1.86 (m, 5H, Pro-βH, Pro-γH, Val-βH), 1.40 (s, 9H, tBu), 0.98 (d, J = 6.7 Hz, 3H, Val-γH), 0.91 (d, J = 6.7 Hz, 3H, Val-γH); 13C NMR (101 MHz, CDCl3) δ 174.5, 172.4, 155.9, 79.6, 59.1, 57.0, 47.5, 31.2, 28.5, 28.3, 24.8, 19.2, 17.6; ESI-MS (m/z): [M+Na]+ calculated for C15H26N2NaO5 337.1739, found 337.1734.
参考文献:
[1] Tetrahedron, 2018, vol. 74, # 12, p. 1184 - 1190
[2] Journal of Organic Chemistry, 2003, vol. 68, # 12, p. 5006 - 5008
[3] Recueil: Journal of the Royal Netherlands Chemical Society, 1980, vol. 99, # 4, p. 124 - 130
[4] Polish Journal of Chemistry, 1988, vol. 62, # 4-6, p. 457 - 464
[5] Bollettino Chimico Farmaceutico, 1999, vol. 138, # 4, p. 160 - 164
