23821-37-6
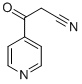 23821-37-6 结构式
23821-37-6 结构式
基本信息
3-羰基-3-吡啶-4基-丙腈
3-氧代-3-(4-吡啶基)丙腈
3-(4-吡啶基)-3-氧代丙腈
3-氧代-3-(4-吡啶基)丙睛
3-氧代-3-(吡啶-4-基)丙腈
b-oxo-4-Pyridinepropanenitrile
4-Pyridinepropanenitrile, β-oxo-
Beta-oxo-4-pyridinepropanenitrile
3-Oxo-3-(4-pyridyl)propanenitrile
3-OXO-3-PYRIDIN-4-YL-PROPIONITRILE
3-OXO-3-PYRIDIN-4-YLPROPANENITRILE
3-OXO-4-PYRIDIN-3-YL-PROPIONITRILE
3-Oxo-3-(4-pyridinyl)propanenitrile
3-oxo-3-(yridine-4-yl)propanenitrile
物理化学性质
制备方法
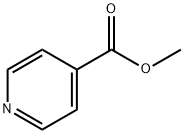
2459-09-8

75-05-8

23821-37-6
以异烟酸甲酯和乙腈为原料,按照路线A1的改进方案合成3-氧代-3-(4-吡啶基)丙腈。具体步骤如下:在氮气保护下,将3g(22mmol)异烟酸甲酯溶解于30mL无水甲苯中,缓慢加入1.75g(44mmol)50-60% NaH(分散于矿物油中)。随后,在90℃条件下缓慢滴加5.39mL(103mmol)干燥乙腈。反应混合物加热回流18小时,期间产物以钠盐形式析出。反应完成后,冷却至室温,过滤收集固体产物。将固体溶于水,用6N HCl溶液调节pH至5-6,用二氯甲烷(DCM)萃取产物。水相再次调节pH至4-5,用DCM二次萃取以回收更多产物。合并有机相,经无水硫酸钠干燥后浓缩,得到粗产物,收率为58%。该粗产物可直接用于后续反应。
参考文献:
[1] Patent: WO2008/87529, 2008, A1. Location in patent: Page/Page column 113
[2] Patent: WO2007/98826, 2007, A2. Location in patent: Page/Page column 47-48
[3] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 24, p. 6890 - 6892
[4] Patent: WO2006/84015, 2006, A2. Location in patent: Page/Page column 86-87
[5] Patent: WO2010/9290, 2010, A1. Location in patent: Page/Page column 143-144
