24190-77-0
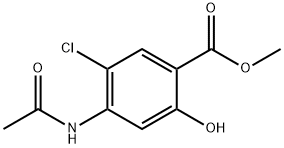 24190-77-0 结构式
24190-77-0 结构式
基本信息
3-氯-4-乙酰氨基水杨酸甲酯
3-氯-4-乙酰胺基水杨酸甲酯
2-羟基-4-乙酰氨基-5-氯苯甲酸甲酯
4-乙酰氨基-5-氯-2-羟基苯甲酸甲酯
5-氯-2-羟基-4-乙酰氨基苯甲酸甲酯
4-乙酰氨基-5-氯-2-羟基苯甲酸甲酯 100G
Methyl 4-acetamido-5-chlorosalicylate
4-acetylamino-5-chloro-2-methoxybenzoate
4-acetylaMino-5-chloro-2-hydroxybenzoate
Methyl 5-chloro-4-acetaMido-2-hydroxybenzoate
Methyl 2-hydroxy-4-acetamido-5-chlorobenzoate
methyl 4-acetamido-2-hydroxy-5-chlorobenzoate
Methyl 4-acetylaMino-5-chloro-2-hdroxybenzoate
METHYL 4-ACETYLAMINO-5-CHLORO-2-HYDROXYBENZOATE
4-Acetamido-5-chloro-2-hydroxybenzoic acid methyl ester
物理化学性质
制备方法
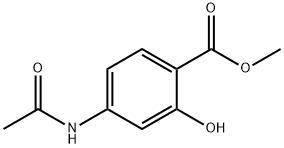
4093-28-1

24190-77-0
以4-乙酰氨基-2-羟基苯甲酸甲酯(61.4克,294.0毫摩尔)为原料,在二氯乙烷(1.2升)中搅拌溶解,随后加入N-氯代琥珀酰亚胺(58.8克,441毫摩尔)。将反应混合物加热回流3小时。反应完成后,通过减压蒸馏去除挥发性溶剂。向残余物中加入水(1.0升)以沉淀固体产物,经过滤收集。粗产物用甲醇与二氯甲烷的混合溶剂(体积比1:9)溶解,并用饱和盐水洗涤。有机层经无水硫酸钠干燥后,减压浓缩,得到4-乙酰氨基-5-氯-2-羟基苯甲酸甲酯(67.7克),产率为94.6%。产物结构经核磁共振氢谱(DMSO-d6)确认:δ10.49(宽单峰,1H),9.47(单峰,1H),7.75(单峰,1H),7.72(单峰,1H),3.85(单峰,3H),2.16(单峰,3H);质谱分析显示分子离子峰(m/z)为244和246(M+H)+。
参考文献:
[1] Patent: WO2013/42135, 2013, A1. Location in patent: Page/Page column 11
[2] Patent: US2014/187581, 2014, A1. Location in patent: Paragraph 0098-0100
[3] Patent: WO2016/128990, 2016, A1. Location in patent: Page/Page column 26-27
[4] Bioorganic and Medicinal Chemistry Letters, 1996, vol. 6, # 22, p. 2657 - 2662
[5] Patent: CN107337658, 2017, A. Location in patent: Paragraph 0043; 0044