24391-41-1
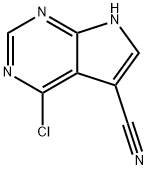 24391-41-1 结构式
24391-41-1 结构式
基本信息
4-氯-7H-吡咯并[2,3-D]嘧啶-5-甲腈
4-氯-7H-吡咯并[2,3-D]嘧啶-5-氰基
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile
7H-Pyrrolo[2,3-d]pyriMidine-5-carbonitrile,4-chloro-
物理化学性质
制备方法
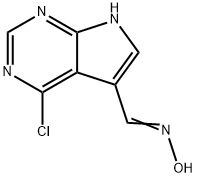
908287-23-0
![4-氯-7H-吡咯[2,3-D]嘧啶-5-甲腈](/CAS/GIF/24391-41-1.gif)
24391-41-1
向4-氯-7H-吡咯并[2,3-d]嘧啶-5-甲醛肟(865 mg,4.40 mmol,1.0当量)在二氯甲烷(20 mL)中的悬浮液中缓慢加入亚硫酰氯(3.1 mL,43.7 mmol,10.0当量)。将反应混合物在室温下搅拌过夜。反应完成后,将混合物在减压下浓缩以除去溶剂。将残余物悬浮于水(60 mL)中,缓慢加入饱和碳酸氢钠水溶液调节pH至4。通过抽滤收集析出的固体,依次用水和乙酸乙酯洗涤,得到第一批产物。随后,用乙酸乙酯(50 mL×3)萃取滤液。合并有机相,用饱和食盐水洗涤,无水硫酸钠干燥,过滤。滤液减压浓缩,所得残余物与前述固体合并。粗产物在乙酸乙酯/己烷(1:1,v/v)混合溶剂中重结晶,减压干燥,得到4-氯-7H-吡咯并[2,3-d]嘧啶-5-甲腈(763 mg,收率97%)。ESI-MS m/z: 178.8 [M + H]+, 176.8 [M - H]+。
参考文献:
[1] Patent: WO2013/12915, 2013, A1. Location in patent: Paragraph 00942
[2] Patent: WO2008/12635, 2008, A2. Location in patent: Page/Page column 83
[3] Patent: US2009/5359, 2009, A1. Location in patent: Page/Page column 25
[4] Patent: WO2011/146882, 2011, A1. Location in patent: Page/Page column 110-111
[5] Patent: US2012/122838, 2012, A1. Location in patent: Page/Page column 81-82
