252723-16-3
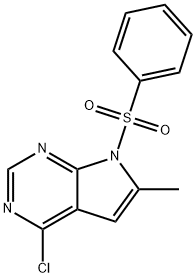 252723-16-3 结构式
252723-16-3 结构式
基本信息
7-苯磺酰基-4-氯-6-甲基-7H-吡咯并[2,3-D]嘧啶
4-氯-6-甲基-7-(苯基磺酰基)-7H-吡咯并[2,3-D]嘧啶
4-Chloro-6-methyl-7-(phenylsulfonyl)-7H-pyrrolo[2,3-d]pyrimidine
7-Benzenesulfonyl-4-chloro-6-methyl-7H-pyrrolo[2,3-d]pyri midine
7H-Pyrrolo[2,3-d]pyrimidine, 4-chloro-6-methyl-7-(phenylsulfonyl)-
物理化学性质
制备方法
![4-氯-7-(苯基磺酰基)-7h-吡咯并[2,3-d]嘧啶](/CAS/GIF/186519-89-1.gif)
186519-89-1

74-88-4
![7-苯磺酰基-4-氯-6-甲基-7H-吡咯并[2,3-D]嘧啶](/CAS/GIF/252723-16-3.gif)
252723-16-3
步骤2:将4-氯-7-(苯磺酰基)-7H-吡咯并[2,3-d]嘧啶(3g,12.6mmol,1.0当量)和N,N,N',N'-四甲基乙二胺(TMEDA)(3.0mL,18.9mmol,1.5当量)溶解于四氢呋喃(THF)(120mL)中,冷却至-78℃。在搅拌下,缓慢加入正丁基锂(n-BuLi)(7.5mL,18.9mmol,1.5当量)。3分钟后,加入碘甲烷(C3/4I)(3.7mL,59.2mmol,4.7当量)。保持反应混合物在-78℃下搅拌3小时,随后在1小时内缓慢升温至室温。反应完成后,用饱和氯化铵(NH4Cl)溶液(10mL)淬灭反应。加入乙酸乙酯(EtOAc)(200mL)和水(100mL)进行萃取。分离有机层,用无水硫酸钠干燥,减压浓缩,得到7-苯磺酰基-4-氯-6-甲基-7H-吡咯并[2,3-d]嘧啶,为棕色固体(6.7g,收率90%)。
参考文献:
[1] Patent: WO2012/158795, 2012, A1. Location in patent: Page/Page column 101-102
[2] Patent: WO2012/158764, 2012, A1. Location in patent: Page/Page column 115
[3] Patent: WO2013/191965, 2013, A1. Location in patent: Page/Page column 191
[4] Patent: WO2014/22569, 2014, A1. Location in patent: Page/Page column 96
[5] Patent: US8673925, 2014, B1. Location in patent: Page/Page column 241