25804-49-3
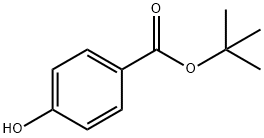 25804-49-3 结构式
25804-49-3 结构式
基本信息
tert-Butyl p-hydroxybenzoate
p-Hydroxybenzoic acid tert-butyl ester
4-Hydroxy-benzoic acid tert-butyl ester
4-Hydroxybenzoic acid 1,1-dimethylethyl ester
Benzoic acid, 4-hydroxy-, 1,1-diMethylethyl ester
物理化学性质
制备方法

75-65-0
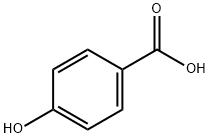
99-96-7

25804-49-3
以对羟基苯甲酸(1.50 g,10.86 mmol)和叔丁醇(13.34 g,18 mmol)为原料,在DBU(0.19 mL,1.20 mmol)催化下,将DCC(2.5 g,12.00 mmol)溶于DCM(40 mL)中,反应混合物在室温下剧烈搅拌18小时。反应完成后,将溶剂蒸发至干,残余物用DCM(50 mL)溶解,过滤除去不溶物。滤液依次用饱和NaHCO3溶液(50 mL)、K2CO3溶液(2×50 mL)和饱和NaCl溶液(50 mL)洗涤。有机相用MgSO4干燥,过滤后蒸发溶剂,得到粗产物。粗产物通过柱色谱法(EtOAc:己烷梯度洗脱)纯化,得到4-羟基苯甲酸叔丁酯(SO2-059)(31),为白色结晶固体(1.1 g,收率55%)。1H NMR(400 MHz,CDCl3)δ 7.89(d,J = 8.8 Hz,1H),6.84(d,J = 8.9 Hz,1H),5.29(s,1H),1.57(s,9H)。
参考文献:
[1] Journal of Materials Chemistry, 2007, vol. 17, # 48, p. 5097 - 5110
[2] Synthesis, 2003, # 16, p. 2479 - 2482
[3] European Journal of Medicinal Chemistry, 2015, vol. 101, p. 716 - 735
[4] Bioorganic and Medicinal Chemistry Letters, 2004, vol. 14, # 14, p. 3645 - 3649
[5] Journal of Medicinal Chemistry, 2013, vol. 56, # 10, p. 3783 - 3805
