263845-82-5
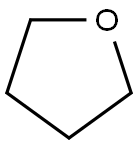
制备方法
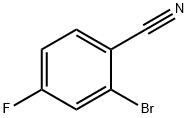
36282-26-5
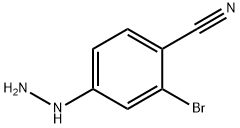
263845-82-5
实施例4a 2-溴-4-肼基苯甲腈的制备:在干燥条件下,通过加料漏斗向2-溴-4-氟苯甲腈(5.00 g,25.00 mmol)的四氢呋喃(THF,10 mL)溶液中缓慢加入无水肼(10 mL)。加料完毕后,将反应混合物于室温下搅拌反应24小时。反应完成后,向混合物中加入水(20 mL)以淬灭反应。通过抽滤收集生成的白色沉淀,并用去离子水充分洗涤。随后,将产物置于40℃的真空烘箱中干燥,得到2-溴-4-肼基苯甲腈(4.92 g,收率93%)为白色固体。产物结构经1H NMR(300 MHz,CDCl3)确认:δ 8.05(br s,1H),7.47(d,J = 8.7 Hz,1H),7.06(d,J = 2.0 Hz,1H),6.72(dd,J = 8.7, 2.0 Hz,1H),4.37(br s,2H);ESI MS m/z 212 [M + H]+。
参考文献:
[1] Patent: US2008/119457, 2008, A1. Location in patent: Page/Page column 45
[2] Patent: WO2006/91963, 2006, A1. Location in patent: Page/Page column 83; 84; 96
[3] Journal of Medicinal Chemistry, 2009, vol. 52, # 14, p. 4288 - 4305
[4] Patent: US2007/207984, 2007, A1. Location in patent: Page/Page column 24
[5] Patent: US2015/329493, 2015, A1. Location in patent: Paragraph 0228; 0242; 0243
